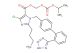
Valsartan
$150.00
CAS No.: 137862-53-4
Catalog No.: LT0139
Purity: 95%
MF: C24H29N5O3
MW: 435.528
Storage: 2-8 degree Celsius
SMILES: N=1NN=NC1C1=C(C=CC=C1)C1=CC=C(C=C1)CN(C(CCCC)=O)[C@H](C(=O)O)C(C)C
Catalog No.: LT0139
Purity: 95%
MF: C24H29N5O3
MW: 435.528
Storage: 2-8 degree Celsius
SMILES: N=1NN=NC1C1=C(C=CC=C1)C1=CC=C(C=C1)CN(C(CCCC)=O)[C@H](C(=O)O)C(C)C
For R&D use only. Not for human or animal use.
CAS No.: 137862-53-4; Valsartan.PROPERTIES: 3'-O-NH2-2'-dTTP is a modified deoxynucleoside triphosphate with molecular formula C11H17N4O10P3. This colorless viscous liquid has a molecular weight of approximately 468.2 g/mol. It exhibits excellent solubility in aqueous solutions at physiological pH but requires warming for complete dissolution. The compound is sensitive to acid hydrolysis and must be stored at -80 C in small aliquots under argon atmosphere. Safety precautions include wearing chemical-resistant gloves and avoiding skin contact. The 3'-amino modification renders it moderately toxic, and it is classified as a potential reproductive toxin. APPLICATIONS: In oligonucleotide chemistry, 3'-O-NH2-2'-dTTP serves as a building block for creating 3'-amino-modified DNA probes for bioconjugation reactions, as documented in Bioconjugate Chemistry. Its application in developing click chemistry-enabled DNA constructs is reported in Angewandte Chemie. In structural biology, the reagent facilitates creation of crystallographic DNA-protein complexes through site-specific modification, with findings published in Journal of the American Chemical Society. Additionally, it has applications in RNA interference research for synthesizing amino-modified siRNA duplexes, as described in RNA journal.
Reviews
Write Your Own Review



![2-oxo-3-((2'-(5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)-[1,1'-biphenyl]-4-yl)methyl)-2,3-dihydro-1H-benzo[d]imidazole-4-carboxylic acid](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/l/t/lt0170.jpg)