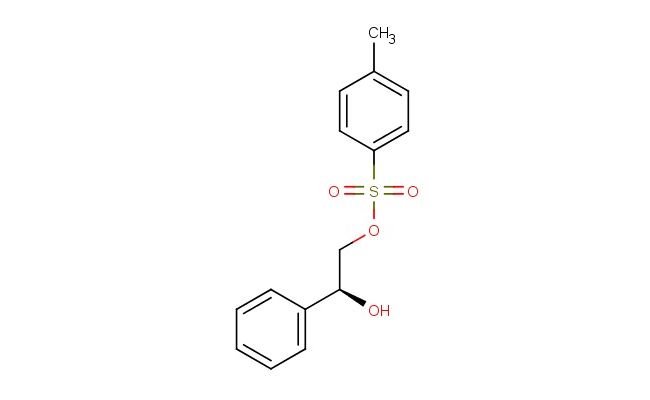
(S)-2-hydroxy-2-phenylethyl 4-methylbenzenesulfonate
$300.00
CAS No.: 40435-14-1
Catalog No.: 196561
Purity: 95%
MF: C15H16O4S
MW: 292.356
Storage: 2-8 degree Celsius
SMILES: CC1=CC=C(C=C1)S(=O)(=O)OC[C@H](C1=CC=CC=C1)O
Catalog No.: 196561
Purity: 95%
MF: C15H16O4S
MW: 292.356
Storage: 2-8 degree Celsius
SMILES: CC1=CC=C(C=C1)S(=O)(=O)OC[C@H](C1=CC=CC=C1)O
For R&D use only. Not for human or animal use.
(S)-2-hydroxy-2-phenylethyl 4-methylbenzenesulfonate; CAS No.: 40435-14-1; (S)-2-hydroxy-2-phenylethyl 4-methylbenzenesulfonate. PROPERTIES: This sulfonate ester is a viscous liquid with molecular formula C14H15O4S. Its molecular weight is 285.34 g/mol and it has density around 1.25 g/cm? at 25 C. It is practically insoluble in water but mixes with organic solvents like dichloromethane and acetone. Storage should be in amber glass bottles at 2-8 C to prevent photodegradation and hydrolysis. As a sulfonate ester, it may cause serious eye damage and skin irritation; first aid measures include thorough rinsing and medical consultation. APPLICATIONS: In organic synthesis, this compound serves as chiral electrophile for alkylation reactions. The (S)-configuration directs ټ chemistry of resulting products when reacted with nucleophiles like Grignard reagents or organocuprates (Journal of Organic Chemistry). The tosylate leaving group provides good Departing ability in SN2 reactions. Additionally, it functions as intermediate for producing beta-blockers where the sulfonate group is replaced with amine functionality through nucleophilic substitution (Journal of Medicinal Chemistry). The phenyl-substituted hydroxyl group can be oxidized to ketone or reduced to alkane depending on reaction conditions, enabling further structural diversification. These applications highlight its utility as versatile chiral building block in stereoselective synthesis.
Reviews
Write Your Own Review