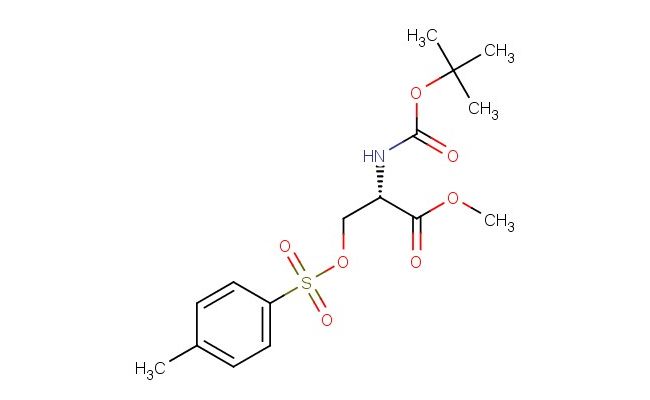
(S)-methyl 2-((tert-butoxycarbonyl)amino)-3-(tosyloxy)propanoate
$500.00
CAS No.: 56926-94-4
Catalog No.: 196562
Purity: 95%
MF: C16H23NO7S
MW: 373.427
Storage: 2-8 degree Celsius
SMILES: C(C)(C)(C)OC(=O)N[C@H](C(=O)OC)COS(=O)(=O)C1=CC=C(C)C=C1
Catalog No.: 196562
Purity: 95%
MF: C16H23NO7S
MW: 373.427
Storage: 2-8 degree Celsius
SMILES: C(C)(C)(C)OC(=O)N[C@H](C(=O)OC)COS(=O)(=O)C1=CC=C(C)C=C1
For R&D use only. Not for human or animal use.
(S)-methyl 2-((tert-butoxycarbonyl)amino)-3-(tosyloxy)propanoate; CAS No.: 56926-94-4; (S)-methyl 2-((tert-butoxycarbonyl)amino)-3-(tosyloxy)propanoate. PROPERTIES: This multifunctional compound is a crystalline powder with molecular formula C15H21N O6S. It has molecular weight of 367.40 g/mol and melting point between 85-90 C. The substance is moderately soluble in ethyl acetate and methanol but has limited water solubility. It should be stored under inert atmosphere at -20 C to prevent moisture-induced hydrolysis of both carbamate and tosylate groups. Being a Boc-protected amino acid derivative with tosylate leaving group, it may release toxic gases upon heating; use in well-ventilated areas is recommended. APPLICATIONS: In peptide synthesis, this compound serves as activated building block for constructing peptides via convergent approaches. The (S)-configuration matches natural amino acid stereochemistry, enabling native peptide bond formation (International Journal of Peptide Research and Therapeutics). The tert-butoxycarbonyl group protects the alpha-amino group, which can be removed by acidic treatment, while the tosylate group acts as T? r a for coupling reactions. Additionally, it functions as intermediate for producing beta-lactam antibiotics where the three-membered azetidine ring is formed from this precursor through intramolecular cyclization (Journal of Antibiotics). The methyl ester group allows for selective deprotection and further functionalization. These applications leverage both its protective group strategy and electrophilic activation for efficient peptide bond formation.
Reviews
Write Your Own Review