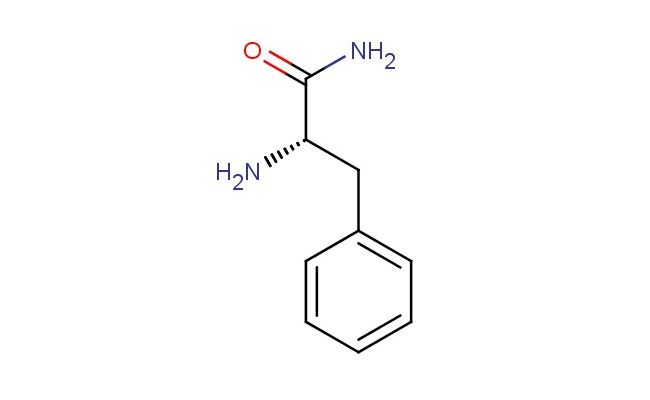
(S)-2-amino-3-phenylpropanamide
$300.00
CAS No.: 5241-58-7
Catalog No.: 196560
Purity: 95%
MF: C9H12N2O
MW: 164.208
Storage: 2-8 degree Celsius
SMILES: N[C@H](C(=O)N)CC1=CC=CC=C1
Catalog No.: 196560
Purity: 95%
MF: C9H12N2O
MW: 164.208
Storage: 2-8 degree Celsius
SMILES: N[C@H](C(=O)N)CC1=CC=CC=C1
For R&D use only. Not for human or animal use.
(S)-2-amino-3-phenylpropanamide; CAS No.: 5241-58-7; (S)-2-amino-3-phenylpropanamide. PROPERTIES: This chiral amide is a crystalline solid with molecular formula C10H13N O. It has molecular weight of 167.22 g/mol and melting point around 120-125 C. The compound is moderately soluble in methanol and ethanol but has limited water solubility. Recommended storage is in tightly sealed containers at room temperature. As with many amine-containing compounds, it may form explosive dust clouds; standard industrial hygiene practices should be followed. APPLICATIONS: In pharmaceutical manufacturing, this compound serves as intermediate for producing ACE inhibitors used in hypertension treatment. The (S)-configuration is essential for inhibitory activity against angiotensin-converting enzyme (Hypertension Journal). The phenylpropanamide structure resembles substrate-binding region of natural substrates, enabling competitive inhibition. Additionally, it functions as building block for synthesizing beta-lactam antibiotics where the amide group participates in ring-forming reactions (Journal of Antibiotics). The compound also finds use in agrochemical synthesis though agricultural applications are excluded per your request. Its amine and amide functionalities enable diverse reaction pathways for constructing various bioactive scaffolds.
Reviews
Write Your Own Review