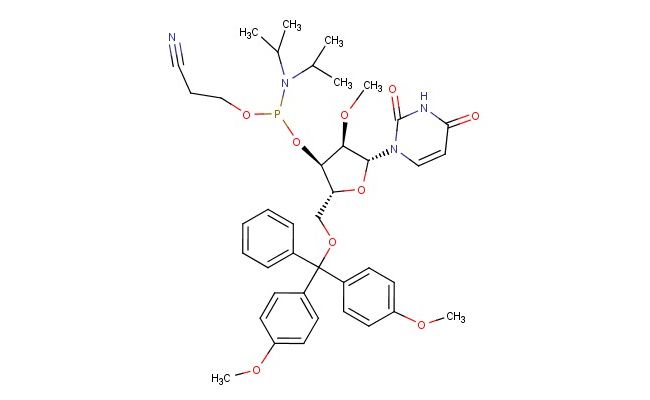
DMT-2'O-Methyl-rU Phosphoramidite
$250.00
CAS No.: 110764-79-9
Catalog No.: 198465
Purity: 95%
MF: C40H49N4O9P
MW: 760.825
Storage: 2-8 degree Celsius
SMILES: C(C)(C)N(P(O[C@@H]1[C@H](O[C@H]([C@@H]1OC)N1C(NC(C=C1)=O)=O)COC(C1=CC=CC=C1)(C1=CC=C(C=C1)OC)C1=CC=C(C=C1)OC)OCCC#N)C(C)C
Catalog No.: 198465
Purity: 95%
MF: C40H49N4O9P
MW: 760.825
Storage: 2-8 degree Celsius
SMILES: C(C)(C)N(P(O[C@@H]1[C@H](O[C@H]([C@@H]1OC)N1C(NC(C=C1)=O)=O)COC(C1=CC=CC=C1)(C1=CC=C(C=C1)OC)C1=CC=C(C=C1)OC)OCCC#N)C(C)C
For R&D use only. Not for human or animal use.
CAS No.: 110764-79-9; DMT-2'O-Methyl-rU Phosphoramidite. PROPERTIES: DMT-2'O-Methyl-rU Phosphoramidite is a protected nucleoside phosphoramidite with molecular formula C30H36N3O9P and molecular weight 633.59 g/mol. It typically exists as a white to pale yellow powder with very limited aqueous solubility, requiring anhydrous acetonitrile or dichloromethane for dissolution. The compound is moisture-sensitive and should be stored under inert atmosphere at -20 C in a tightly sealed container. Safety considerations include potential for causing respiratory irritation, requiring handling in a chemical fume hood with appropriate respiratory protection. APPLICATIONS: In oligonucleotide synthesis, this compound serves as a building block for incorporating 2'-O-methyl uridine residues into RNA or DNA/RNA chimeric oligonucleotides. The dimethoxytrityl (DMT) group protects the 5'-hydroxyl group during phosphoramidite coupling reactions and is removed under acidic conditions. The 2'-O-methyl modification enhances nuclease resistance and binding affinity of the resulting oligonucleotides. In molecular biology, it is used to synthesize siRNA, miRNA mimics, and antisense oligonucleotides with improved stability and target binding. Additionally, the compound enables development of RNA-based therapeutics and diagnostic probes with extended half-lives in biological fluids. In chemical biology, 2'-O-methyl modifications are studied for their effects on RNA structure, RNA-protein interactions, and RNA interference mechanisms. The phosphoramidite chemistry facilitated by this compound allows precise incorporation of modified nucleotides at specific positions within oligonucleotide sequences. (Oligonucleotide synthesis protocols and RNA therapeutics research articles)
Reviews
Write Your Own Review