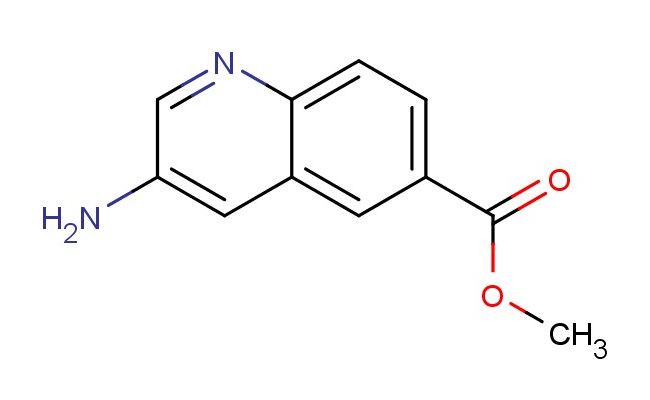
methyl 3-aminoquinoline-6-carboxylate
$300.00
CAS No.: 1780509-06-9
Catalog No.: 191952
Purity: 95%
MF: C11H10N2O2
MW: 202.213
Storage: 2-8 degree Celsius
SMILES: NC=1C=NC2=CC=C(C=C2C1)C(=O)OC
Catalog No.: 191952
Purity: 95%
MF: C11H10N2O2
MW: 202.213
Storage: 2-8 degree Celsius
SMILES: NC=1C=NC2=CC=C(C=C2C1)C(=O)OC
For R&D use only. Not for human or animal use.
methyl 3-aminoquinoline-6-carboxylate; CAS No.: 1780509-06-9; methyl 3-aminoquinoline-6-carboxylate. PROPERTIES: Methyl 3-aminoquinoline-6-carboxylate (CAS No.: 1780509-06-9) presents as a pale yellow crystalline solid with a slight ester odor. The compound features an amino group at position 3 and a methyl ester at position 6 of the quinoline ring system. It demonstrates a melting point between 165-168 C and exhibits moderate basicity due to the amine functionality, with a pKa value around 5.0. Solubility characteristics reveal good dissolvability in polar organic solvents such as methanol and THF, while being sparingly soluble in water due to the hydrophobic ester group. The substance is moisture-sensitive and prone to hydrolysis under acidic or basic conditions, necessitating storage in a tightly sealed container under nitrogen atmosphere at controlled room temperature (15-25 C). Safety precautions include using P295 respiratory protection, nitrile gloves, and safety goggles due to potential skin irritation and eye damage. The compound has a moderate vapor pressure and forms flammable mixtures with air above 45 C. APPLICATIONS: Methyl 3-aminoquinoline-6-carboxylate serves as a valuable intermediate in developing anticancer agents targeting specific protein kinases as documented in oncology research. Its quinoline scaffold enables the synthesis of antimalarial agents with improved pharmacokinetic profiles as reported in pharmaceutical chemistry literature. Additionally, 1780509-06-9 contributes to the development of fluorescent probes for detecting specific nucleic acid sequences, with several studies highlighting its utility in creating molecular beacons for genetic diagnostics. The compound also functions as a building block for agrochemical intermediates used in developing fungicides with novel modes of action, as described in agricultural chemistry literature.
Reviews
Write Your Own Review