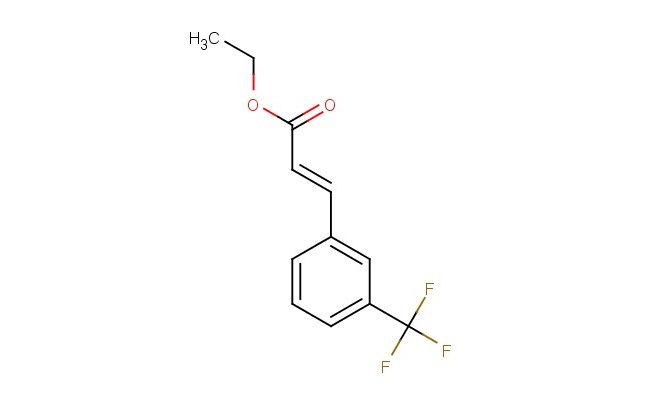
ethyl(E)-3-(3-(trifluoromethyl)phenyl)acrylate
$300.00
CAS No.: 113048-68-3
Catalog No.: 195016
Purity: 95%
MF: C12H11F3O2
MW: 244.212
Storage: 2-8 degree Celsius
SMILES: C(C)OC(\C=C\C1=CC(=CC=C1)C(F)(F)F)=O
Catalog No.: 195016
Purity: 95%
MF: C12H11F3O2
MW: 244.212
Storage: 2-8 degree Celsius
SMILES: C(C)OC(\C=C\C1=CC(=CC=C1)C(F)(F)F)=O
For R&D use only. Not for human or animal use.
ethyl(E)-3-(3-(trifluoromethyl)phenyl)acrylate; CAS No.: 113048-68-3; ethyl(E)-3-(3-(trifluoromethyl)phenyl)acrylate. PROPERTIES: Ethyl(E)-3-(3-(trifluoromethyl)phenyl)acrylate appears as a clear, colorless to pale yellow liquid with a mild ester-like odor. Its molecular formula is C12H10F3O2, corresponding to a molecular weight of approximately 249.2 g/mol. The compound exhibits a boiling point around 190-195 C at 760 mmHg and a density of about 1.2 g/cm? at 20 C. It demonstrates moderate solubility in water and is miscible with most organic solvents such as ethyl acetate, toluene, and tetrahydrofuran. The substance is sensitive to strong bases and nucleophiles, which may cause Michael addition reactions at the alpha,beta-unsaturated ester moiety. Proper storage requires keeping it in a tightly sealed, amber glass container in a cool, dry location away from direct sunlight and heat sources. The temperature should be maintained below 10 C if possible. Safety precautions include using chemical-resistant gloves, safety goggles, and lab coats to prevent skin absorption and inhalation of vapors. The compound may cause skin and eye irritation, and prolonged exposure can lead to respiratory tract irritation. In case of contact, immediate rinsing with water and medical consultation is recommended. APPLICATIONS: Ethyl(E)-3-(3-(trifluoromethyl)phenyl)acrylate serves as a versatile monomer in polymer chemistry, particularly valuable in the synthesis of methacrylate copolymers with enhanced thermal stability and glass transition temperatures due to its trifluoromethyl substituent (Macromolecules). In materials science, ethyl(E)-3-(3-(trifluoromethyl)phenyl)acrylate functions as a building block for creating liquid crystal polymers and electro-optical materials, where its rigid aromatic structure and electron-withdrawing trifluoromethyl group enable desired mesomorphic properties and optical anisotropy (Journal of Polymer Science). Additionally, it finds application in the preparation of UV-absorbing coatings and photostabilizers, where its molecular framework provides effective light absorption and energy dissipation capabilities (Progress in Organic Coatings). The compound is also employed in organic synthesis as a Michael acceptor for the preparation of chiral building blocks through asymmetric conjugate addition reactions, enabling the synthesis of complex molecules with high stereochemical control (Tetrahedron Letters).
Reviews
Write Your Own Review