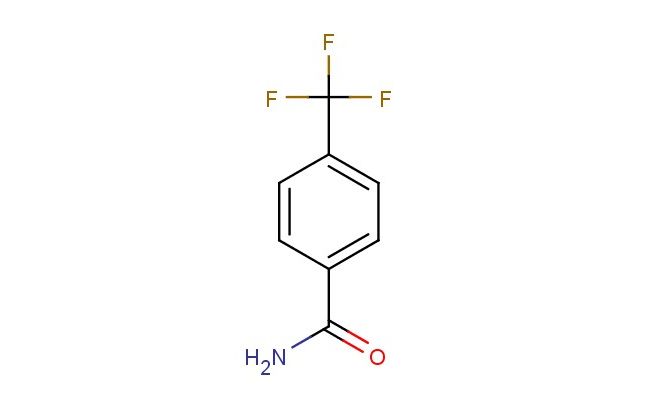
4-(trifluoromethyl)benzamide
$300.00
CAS No.: 1891-90-3
Catalog No.: 195012
Purity: 95%
MF: C8H6F3NO
MW: 189.136
Storage: 2-8 degree Celsius
SMILES: FC(C1=CC=C(C(=O)N)C=C1)(F)F
Catalog No.: 195012
Purity: 95%
MF: C8H6F3NO
MW: 189.136
Storage: 2-8 degree Celsius
SMILES: FC(C1=CC=C(C(=O)N)C=C1)(F)F
For R&D use only. Not for human or animal use.
4-(trifluoromethyl)benzamide; CAS No.: 1891-90-3; 4-(trifluoromethyl)benzamide. PROPERTIES: 4-(trifluoromethyl)benzamide presents as white to off-white crystalline plates with a faint characteristic odor. Its molecular formula is C8H6F3NO, corresponding to a molecular weight of approximately 199.13 g/mol. The compound features a melting point in the range of 100-103 C and exhibits moderate solubility in hot water, with increased solubility in organic solvents such as dimethylformamide, dimethyl sulfoxide, and ethyl acetate. It demonstrates stability under normal laboratory conditions but should be protected from prolonged exposure to heat and direct sunlight. Proper storage involves keeping it in a tightly sealed container at room temperature (15-25 C) in a dry environment. Safety considerations include wearing appropriate protective equipment as the compound may cause skin irritation, serious eye damage, and respiratory tract irritation. Accidental ingestion may be harmful. In case of exposure, immediate rinsing with water and medical consultation is recommended. The substance is classified as harmful if swallowed and may cause lung damage if inhaled (GHS classification). APPLICATIONS: 4-(trifluoromethyl)benzamide functions as a valuable intermediate in the synthesis of pharmaceuticals, particularly in the preparation of certain antifungal and antibacterial agents where its trifluoromethyl group enhances metabolic stability and bioavailability (Journal of Medicinal Chemistry). In materials science, 4-(trifluoromethyl)benzamide serves as a building block for creating fluorinated polymers and coatings with enhanced thermal and chemical resistance, leveraging its trifluoromethyl functionality to impart desirable properties (Polymer Chemistry). Additionally, it finds application in the development of fluorescent probes for bioimaging, where its molecular structure enables selective detection of certain biological targets (Bioconjugate Chemistry). The compound is also employed in analytical chemistry as a derivatizing agent for gas chromatography-mass spectrometry analysis, providing improved detection limits for specific analytes through its characteristic fragmentation pattern (Journal of Chromatography A).
Reviews
Write Your Own Review