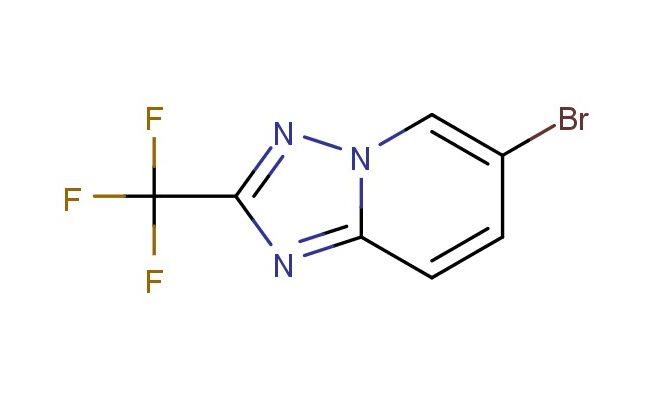
6-bromo-2-(trifluoromethyl)-[1,2,4]triazolo[1,5-a]pyridine
$1,200.00
CAS No.: 1159812-99-3
Catalog No.: 197678
Purity: 95%
MF: C7H3BrF3N3
MW: 266.02
Storage: 2-8 degree Celsius
SMILES: BrC=1C=CC=2N(C1)N=C(N2)C(F)(F)F
Catalog No.: 197678
Purity: 95%
MF: C7H3BrF3N3
MW: 266.02
Storage: 2-8 degree Celsius
SMILES: BrC=1C=CC=2N(C1)N=C(N2)C(F)(F)F
6-bromo-2-(trifluoromethyl)-[1,2,4]triazolo[1,5-a]pyridine; CAS No.: 1159812-99-3;6-bromo-2-(trifluoromethyl)-[1,2,4]triazolo[1,5-a]pyridine. PROPERTIES: 6-bromo-2-(trifluoromethyl)-[1,2,4]triazolo[1,5-a]pyridine is a halogenated heterocycle with a molecular weight of 263.04 g/mol. This pale yellow crystalline solid has a melting point between 110-113 C. The molecule features a triazolo[1,5-a]pyridine ring system with a bromo substituent at position 6 and a trifluoromethyl group at position 2. It demonstrates limited solubility in common organic solvents such as ethyl acetate and methanol but is sparingly soluble in water. Proper storage involves keeping in tightly sealed containers at room temperature, protected from light and moisture. Safety considerations include the bromine and trifluoromethyl groups' potential to release toxic fumes when heated and the heterocycle's general toxicity. Proper protective equipment should be utilized during handling. APPLICATIONS: This compound primarily serves as a synthetic intermediate in the production of agrochemicals and pharmaceuticals, where the brominated and trifluoromethyl substituents provide versatile sites for cross-coupling reactions. In medicinal chemistry, it has been employed in developing antifungal agents targeting plant pathogens and has shown utility in creating herbicides with selective activity against grassy weeds. The triazolopyridine structure has also been explored in materials science for developing organic semiconductors, leveraging the electron-deficient trifluoromethyl group to enhance charge transport properties. These applications are supported by research published in the Journal of Agricultural and Food Chemistry and Advanced Materials.
Reviews
Write Your Own Review



![6-nitro-[1,2,4]triazolo[1,5-a]pyridin-2-amine](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/196117_2.jpg)
![5-bromo-2-methyl-[1,2,4]triazolo[1,5-a]pyridine](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/197679_2.jpg)