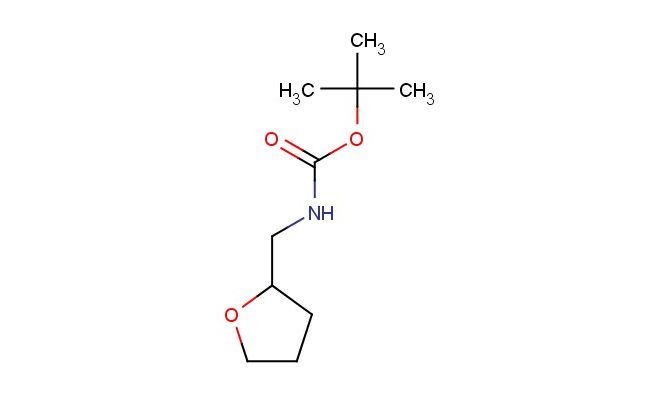
tert-butyl ((tetrahydrofuran-2-yl)methyl)carbamate
$180.00
CAS No.: 274901-68-7
Catalog No.: 192581
Purity: 95%
MF: C10H19NO3
MW: 201.266
Storage: 2-8 degree Celsius
SMILES: O1C(CCC1)CNC(OC(C)(C)C)=O
Catalog No.: 192581
Purity: 95%
MF: C10H19NO3
MW: 201.266
Storage: 2-8 degree Celsius
SMILES: O1C(CCC1)CNC(OC(C)(C)C)=O
tert-butyl ((tetrahydrofuran-2-yl)methyl)carbamate; CAS No.: 274901-68-7; tert-butyl ((tetrahydrofuran-2-yl)methyl)carbamate. PROPERTIES: tert-butyl ((tetrahydrofuran-2-yl)methyl)carbamate is a crystalline solid with a molecular weight of 201.27 g/mol. It has a melting point ranging from 45-50 C and a boiling point of approximately 180-190 C at 760 mmHg. The compound is moderately soluble in polar organic solvents like dichloromethane and dimethylformamide but has limited water solubility. It is sensitive to acidic conditions and hydrolyzes in the presence of strong acids to release the corresponding amine. Storage should be in a sealed container at temperatures below 20 C, protected from moisture and acidic vapors. Safety measures include avoiding ingestion and skin contact, as it may cause gastrointestinal upset and dermal irritation. In case of eye contact, immediate flushing with water for 15 minutes is recommended. This compound should be handled in well-ventilated areas to prevent accumulation of vapors, which might pose an inhalation hazard. APPLICATIONS: tert-butyl ((tetrahydrofuran-2-yl)methyl)carbamate is predominantly used as a protected amine building block in peptide synthesis. Its Boc protection allows for selective deprotection under acidic conditions, making it useful in solid-phase peptide synthesis as outlined in organic chemistry protocols. In pharmaceutical development, it serves as an intermediate for beta-blockers where the tetrahydrofuran ring enhances membrane permeability. For example, it can be employed in synthesizing carvedilol analogs, where the carbamate protection strategy facilitates multi-step synthesis without premature amine reactions as described in medicinal chemistry research. Additionally, it finds utility in material science as a chiral building block for liquid crystal polymers, where the tetrahydrofuran moiety contributes to mesomorphic properties, as reported in polymer chemistry studies. The compound also acts as a precursor for agrochemicals like certain fungicides, where the protected amine functionality is later revealed to interact with fungal enzyme active sites, as detailed in agrochemical formulation literature.
Reviews
Write Your Own Review