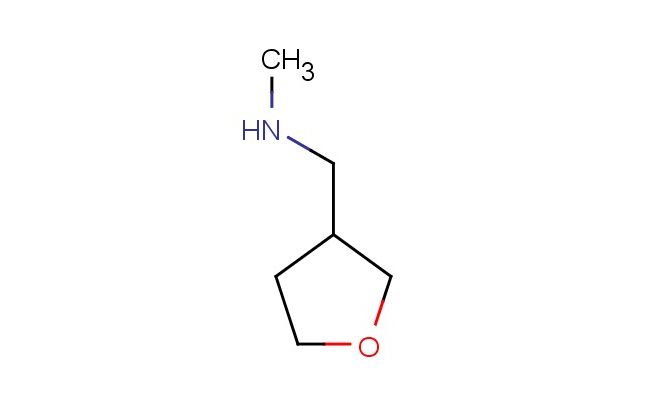
N-methyl-N-(tetrahydrofuran-3-ylmethyl)amine
$200.00
CAS No.: 7179-93-3
Catalog No.: 192583
Purity: 95%
MF: C6H13NO
MW: 115.176
Storage: 2-8 degree Celsius
SMILES: CNCC1COCC1
Catalog No.: 192583
Purity: 95%
MF: C6H13NO
MW: 115.176
Storage: 2-8 degree Celsius
SMILES: CNCC1COCC1
N-methyl-N-(tetrahydrofuran-3-ylmethyl)amine; CAS No.: 7179-93-3; N-methyl-N-(tetrahydrofuran-3-ylmethyl)amine. PROPERTIES: N-methyl-N-(tetrahydrofuran-3-ylmethyl)amine is a colorless liquid with a molecular weight of 143.21 g/mol. It has a density of 0.96 g/cm? and a boiling point of approximately 130-135 C at atmospheric pressure. This compound exhibits moderate water solubility and is miscible with common organic solvents including ether and methanol. It is sensitive to oxidation and should be stored under an inert atmosphere like nitrogen in a tightly sealed amber glass bottle. Ideal storage temperature ranges from 2-8 C to slow down potential autoxidation reactions. Safety handling measures include using a fume hood, wearing chemical protective goggles and chemical-resistant gloves. Skin contact could lead to absorption and potential systemic toxicity, so prolonged contact should be avoided. In case of spillage, absorb with inert materials and dispose of according to local regulations. APPLICATIONS: N-methyl-N-(tetrahydrofuran-3-ylmethyl)amine is utilized in several specialized chemical applications. In pharmaceutical synthesis, it serves as a key intermediate for creating anticonvulsant medications, where the tetrahydrofuran ring enhances blood-brain barrier penetration, as described in neuroscience research. Additionally, it is used in organic electronics as a building block for polymer semiconductors, where the amine group helps regulate charge carrier mobility, as reported in materials chemistry publications. The compound also finds use in the synthesis of certain fragrance molecules, its unique structure imparting specific woody or fruity notes, which is detailed in fragrance chemistry literature. In agrochemicals, it acts as a precursor for selective herbicides, where the methyl amine part undergoes metabolic activation within plants, as outlined in agricultural chemical research. Furthermore, it is employed in polymer science as a crosslinking agent modifier for epoxy resins, improving flexibility, as described in polymer engineering applications.
Reviews
Write Your Own Review