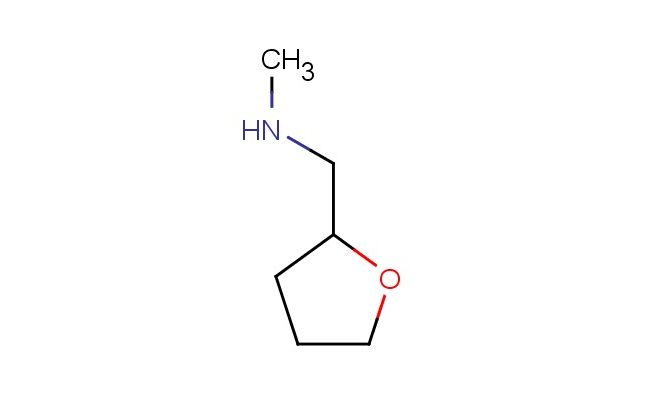
N-methyl-1-(tetrahydrofuran-2-yl)methanamine
$200.00
CAS No.: 2439-57-8
Catalog No.: 192580
Purity: 95%
MF: C6H13NO
MW: 115.176
Storage: 2-8 degree Celsius
SMILES: CNCC1OCCC1
Catalog No.: 192580
Purity: 95%
MF: C6H13NO
MW: 115.176
Storage: 2-8 degree Celsius
SMILES: CNCC1OCCC1
N-methyl-1-(tetrahydrofuran-2-yl)methanamine; CAS No.: 2439-57-8; N-methyl-1-(tetrahydrofuran-2-yl)methanamine. PROPERTIES: N-methyl-1-(tetrahydrofuran-2-yl)methanamine appears as a colorless to pale yellow liquid with a slight amine odor. It has a molecular weight of 129.19 g/mol, a density of 0.98 g/cm?, and a boiling point of approximately 115-120 C at 760 mmHg. This compound is moderately soluble in water and miscible with organic solvents like ethanol and acetone. It is hygroscopic and sensitive to air and light. Proper storage requires a tightly sealed container in a cool, dry, and well-ventilated area away from oxidizing agents and acidic materials. Safety precautions include using personal protective equipment such as gloves, eye protection, and lab coats. It should be handled in a chemical fume hood to prevent inhalation of vapors, which may cause respiratory irritation. Skin contact can lead to absorption and potential systemic toxicity. In case of spillage, absorb with inert material and dispose of according to local regulations. APPLICATIONS: N-methyl-1-(tetrahydrofuran-2-yl)methanamine serves as a valuable intermediate in organic synthesis. In pharmaceutical research, it is used to synthesize compounds with potential central nervous system activity. For instance, it can be utilized in the preparation of certain antipsychotic drugs where the tetrahydrofuran ring contributes to receptor binding affinity as described in medicinal chemistry literature. Additionally, it finds application in agrochemical formulations as a building block for herbicides targeting specific plant enzymes. The amine functionality allows for further derivatization in peptide synthesis, particularly in creating unnatural amino acid analogs for protein engineering studies as reported in bioorganic chemistry journals. Its reactivity makes it suitable for resin chemistry in polymer science, where it can be incorporated into crosslinking agents for epoxy systems, enhancing adhesion properties as detailed in polymer chemistry publications.
Reviews
Write Your Own Review