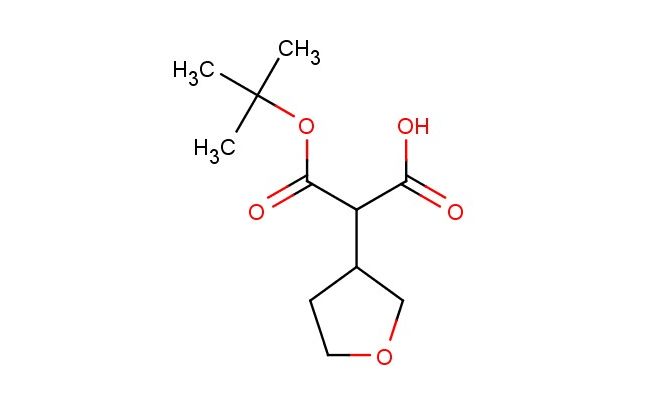
2-(tert-butoxycarbonyl)-2-(tetrahydrofuran-3-yl)acetic acid
$240.00
CAS No.: 874583-03-6
Catalog No.: 192584
Purity: 95%
MF: C11H18O5
MW: 230.26
Storage: 2-8 degree Celsius
SMILES: C(C)(C)(C)OC(=O)C(C(=O)O)C1COCC1
Catalog No.: 192584
Purity: 95%
MF: C11H18O5
MW: 230.26
Storage: 2-8 degree Celsius
SMILES: C(C)(C)(C)OC(=O)C(C(=O)O)C1COCC1
2-(tert-butoxycarbonyl)-2-(tetrahydrofuran-3-yl)acetic acid; CAS No.: 874583-03-6; 2-(tert-butoxycarbonyl)-2-(tetrahydrofuran-3-yl)acetic acid. PROPERTIES: 2-(tert-butoxycarbonyl)-2-(tetrahydrofuran-3-yl)acetic acid presents as a white crystalline powder with a molecular weight of 255.28 g/mol. It has a melting point between 120-125 C and is moderately soluble in polar aprotic solvents such as dimethylformamide and dimethyl sulfoxide. The compound is sensitive to acidic and basic conditions, undergoing hydrolysis when exposed to strong acids or bases. Proper storage involves keeping it in a desiccator with drying agents at temperatures below 25 C. Safety considerations include wearing protective eyewear and laboratory coats during handling to prevent eye irritation and skin contact. In case of accidental ingestion, immediate medical attention is advisable. The compound should be stored away from heat sources and incompatible materials like strong oxidizers. APPLICATIONS: 2-(tert-butoxycarbonyl)-2-(tetrahydrofuran-3-yl)acetic acid is primarily employed as a protected amino acid derivative in peptide synthesis. Its Boc protection allows for orthogonal protection strategies in complex peptide assembly, as detailed in organic synthesis textbooks. In pharmaceutical research, it serves as a building block for creating beta-lactamase inhibitors, where the tetrahydrofuran ring contributes to enzyme binding, as described in medicinal chemistry literature. Additionally, it finds application in the creation of chiral auxiliaries for asymmetric synthesis, where the tetrahydrofuran substituent induces stereocontrol during chemical reactions, as reported in stereochemistry journals. The compound is also utilized in material science for creating bioabsorbable polymers, where the Boc group can be removed under specific conditions to trigger polymer degradation, as outlined in biomedical materials research. Furthermore, it acts as a precursor for certain agrochemicals, particularly in the synthesis of plant growth regulators where the controlled release of the carboxylic acid functionality is crucial for efficacy, as detailed in agricultural chemistry publications.
Reviews
Write Your Own Review