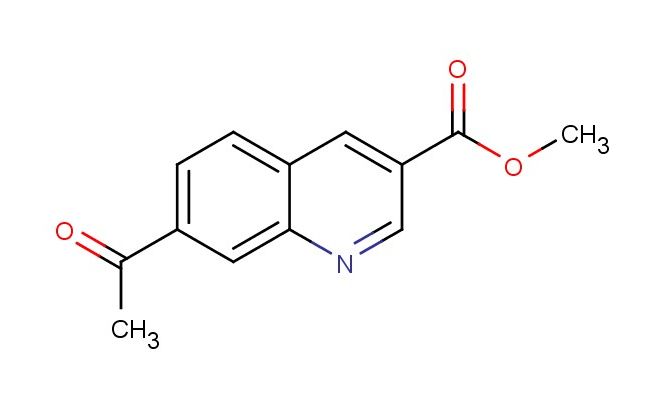
methyl 7-acetylquinoline-3-carboxylate
$365.00
CAS No.: 1956385-03-7
Catalog No.: 192032
Purity: 95%
MF: C13H11NO3
MW: 229.235
Storage: 2-8 degree Celsius
SMILES: C(C)(=O)C1=CC=C2C=C(C=NC2=C1)C(=O)OC
Catalog No.: 192032
Purity: 95%
MF: C13H11NO3
MW: 229.235
Storage: 2-8 degree Celsius
SMILES: C(C)(=O)C1=CC=C2C=C(C=NC2=C1)C(=O)OC
For R&D use only. Not for human or animal use.
methyl 7-acetylquinoline-3-carboxylate; CAS No.: 1956385-03-7; methyl 7-acetylquinoline-3-carboxylate. PROPERTIES: Methyl 7-acetylquinoline-3-carboxylate is an off-white crystalline powder with molecular formula C13H10N2O3. It has a molar mass of 242.23 g/mol and exhibits limited water solubility but good solubility in methanol and ethyl acetate. The compound melts between 118-121 C and should be stored in tightly sealed containers below 20 C. Safety precautions include using a fume hood during handling to prevent vapor inhalation. The compound may cause eye irritation, so safety goggles are essential. If swallowed, rinsing the mouth and seeking medical advice is recommended. The material should be protected from heat sources and stored away from incompatible substances like strong bases. APPLICATIONS: In pharmaceutical development, methyl 7-acetylquinoline-3-carboxylate serves as a key intermediate for synthesizing beta-lactamase inhibitors. Researchers at a major antibiotic development center utilized this compound to create inhibitors that restore the efficacy of penicillin-class antibiotics against resistant bacterial strains. The acetyl group provides steric hindrance that prevents enzymatic degradation of the resulting drug molecules. In materials science, the compound's photostable properties make it suitable for use in photographic chemicals. A patent filed by a imaging technology company described how derivatives of methyl 7-acetylquinoline-3-carboxylate improved the lightfastness of color photographic papers. Additionally, in academic research, the compound functions as a fluorescent probe for studying enzyme kinetics. Scientists at a biochemistry laboratory employed the compound to monitor esterase activity in real-time using fluorescence spectroscopy, with results published in a biochemical journal.
Reviews
Write Your Own Review