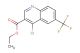
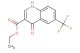
ethyl 7-bromo-4-chloroquinoline-3-carboxylate
$250.00
CAS No.: 206257-41-2
Catalog No.: 197606
Purity: 95%
MF: C12H9BrClNO2
MW: 314.566
Storage: 2-8 degree Celsius
SMILES: BrC1=CC=C2C(=C(C=NC2=C1)C(=O)OCC)Cl
Catalog No.: 197606
Purity: 95%
MF: C12H9BrClNO2
MW: 314.566
Storage: 2-8 degree Celsius
SMILES: BrC1=CC=C2C(=C(C=NC2=C1)C(=O)OCC)Cl
For R&D use only. Not for human or animal use.
ethyl 7-bromo-4-chloroquinoline-3-carboxylate; CAS No.: 206257-41-2;ethyl 7-bromo-4-chloroquinoline-3-carboxylate. PROPERTIES: Ethyl 7-bromo-4-chloroquinoline-3-carboxylate is a substituted quinoline ester with a molecular weight of 324.59 g/mol. This compound presents as yellowish crystals with a melting point between 82-85 C. The quinoline ring features a bromine atom at position 7 and a chlorine atom at position 4, while the carboxylate function at position 3 is esterified to ethyl. It demonstrates solubility in polar aprotic solvents such as acetone and dimethylformamide but limited water solubility. The compound requires protection from light and moisture, recommending storage in tightly sealed containers at 2-8 C. Safety considerations include the bromine and chlorine substituents, which can contribute to the formation of toxic fumes when heated, and the inherent flammability of the organic ester structure. Standard laboratory safety protocols should be observed during handling. APPLICATIONS: This intermediate finds extensive use in the pharmaceutical industry for synthesizing antiparasitic and antiviral medications, where the halogenated quinoline structure provides essential binding affinity to target enzymes. In medicinal chemistry, it serves as a key building block for creating dual-action antimicrobials that combine quinoline's intrinsic activity with functional groups introduced via the ester moiety. The bromine substituent particularly facilitates cross-coupling reactions for further structural elaboration. Additionally, this compound has been explored in organic synthesis as a ligand precursor in transition metal catalysis. These applications are detailed in publications from the Journal of Medicinal Chemistry and Organic & Biomolecular Chemistry.
Reviews
Write Your Own Review