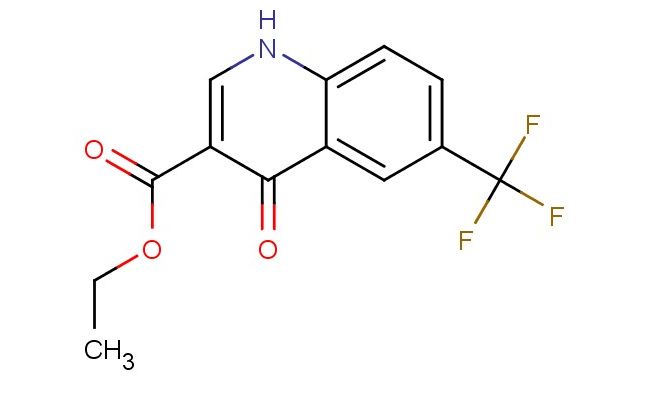
ethyl 4-oxo-6-(trifluoromethyl)-1,4-dihydroquinoline-3-carboxylate
$250.00
CAS No.: 26893-12-9
Catalog No.: 197607
Purity: 95%
MF: C13H10F3NO3
MW: 285.221
Storage: 2-8 degree Celsius
SMILES: O=C1C(=CNC2=CC=C(C=C12)C(F)(F)F)C(=O)OCC
Catalog No.: 197607
Purity: 95%
MF: C13H10F3NO3
MW: 285.221
Storage: 2-8 degree Celsius
SMILES: O=C1C(=CNC2=CC=C(C=C12)C(F)(F)F)C(=O)OCC
For R&D use only. Not for human or animal use.
ethyl 4-oxo-6-(trifluoromethyl)-1,4-dihydroquinoline-3-carboxylate; CAS No.: 26893-12-9;ethyl 4-oxo-6-(trifluoromethyl)-1,4-dihydroquinoline-3-carboxylate. PROPERTIES: Ethyl 4-oxo-6-(trifluoromethyl)-1,4-dihydroquinoline-3-carboxylate is a dihydronaphthyridine derivative with a molecular weight of 314.29 g/mol. This off-white powder exhibits a characteristic melting point of 128-131 C. The compound features a partially reduced quinoline ring system with an oxo group at position 4, a trifluoromethyl substituent at position 6, and an ethyl ester at position 3. It shows moderate solubility in methanol and ethyl acetate while being sparingly soluble in water. Storage requirements include maintaining in airtight containers away from heat sources and direct sunlight, preferably at ambient temperature. Safety precautions center around the trifluoromethyl group's potential to release hydrofluoric acid upon pyrolysis and the ketone functionality's moderate toxicity. Standard protective measures for handling chemical intermediates should be employed. APPLICATIONS: This compound primarily functions as a synthetic intermediate in the production of cardiovascular medications and antimicrobial agents, where the oxo and trifluoromethyl groups enhance metabolic stability and receptor binding. In pharmaceutical development, it serves as a precursor to beta-lactamase inhibitors and has been explored in the synthesis of HIV integrase inhibitors. The dihydronaphthyridine scaffold has also garnered interest in materials science for developing electroluminescent materials, leveraging the electron-withdrawing trifluoromethyl group to tune optical properties. These applications are documented in the European Journal of Medicinal Chemistry and Advanced Functional Materials.
Reviews
Write Your Own Review