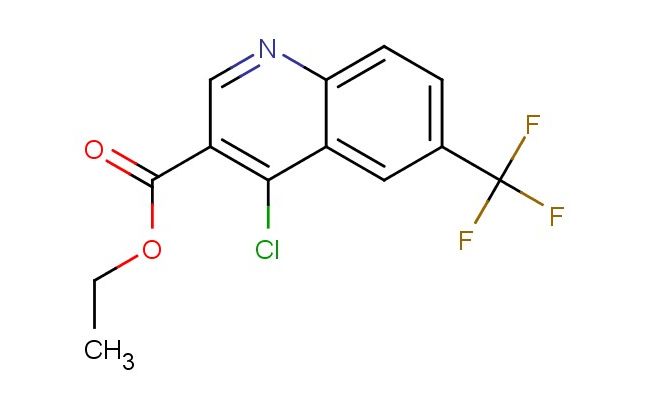
ethyl 4-chloro-6-(trifluoromethyl)quinoline-3-carboxylate
$400.00
CAS No.: 193827-69-9
Catalog No.: 197605
Purity: 95%
MF: C13H9ClF3NO2
MW: 303.667
Storage: 2-8 degree Celsius
SMILES: ClC1=C(C=NC2=CC=C(C=C12)C(F)(F)F)C(=O)OCC
Catalog No.: 197605
Purity: 95%
MF: C13H9ClF3NO2
MW: 303.667
Storage: 2-8 degree Celsius
SMILES: ClC1=C(C=NC2=CC=C(C=C12)C(F)(F)F)C(=O)OCC
For R&D use only. Not for human or animal use.
ethyl 4-chloro-6-(trifluoromethyl)quinoline-3-carboxylate; CAS No.: 193827-69-9;ethyl 4-chloro-6-(trifluoromethyl)quinoline-3-carboxylate. PROPERTIES: Ethyl 4-chloro-6-(trifluoromethyl)quinoline-3-carboxylate is an esterified quinoline derivative with a molecular weight of 312.73 g/mol. This pale yellow crystalline solid features a quinoline ring bearing a chloro substituent at position 4 and a trifluoromethyl group at position 6, with the carboxylate function esterified to ethyl at position 3. Its physical characteristics include a melting point of approximately 68-70 C and moderate solubility in common organic solvents like ethyl acetate and tetrahydrofuran while being insoluble in water. The compound is sensitive to moisture and light, necessitating storage in amber glass containers with tight seals at temperatures below 20 C. Safety considerations involve its trifluoromethyl group, which can produce toxic fumes when exposed to high heat, and the chloro substituent that may form phosgene under combustion conditions. Standard precautions include working in well-ventilated areas and using personal protective equipment. APPLICATIONS: This compound primarily functions as a synthetic intermediate in pharmaceutical manufacturing, particularly in the production of antimalarial and antibacterial agents where the trifluoromethyl and chloro substituents enhance lipophilicity and cellular penetration. In agrochemical development, it serves as a building block for creating selective herbicides targeting specific enzyme systems in plants. The quinoline scaffold has also gained attention in materials science for developing fluorescent probes and sensors, leveraging the unique electronic properties conferred by the trifluoromethyl group. These applications are supported by research published in Organic Process Research & Development and the Journal of Agricultural and Food Chemistry.
Reviews
Write Your Own Review