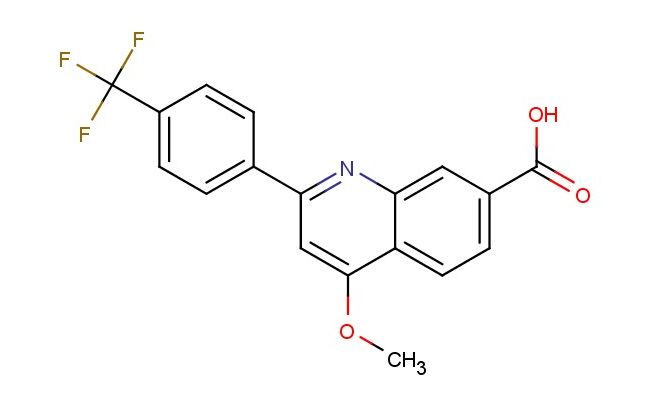
4-methoxy-2-(4-(trifluoromethyl)phenyl)quinoline-7-carboxylic acid
$200.00
CAS No.: 2842025-72-1
Catalog No.: 197608
Purity: 95%
MF: C18H12F3NO3
MW: 347.292
Storage: 2-8 degree Celsius
SMILES: COC1=CC(=NC2=CC(=CC=C12)C(=O)O)C1=CC=C(C=C1)C(F)(F)F
Catalog No.: 197608
Purity: 95%
MF: C18H12F3NO3
MW: 347.292
Storage: 2-8 degree Celsius
SMILES: COC1=CC(=NC2=CC(=CC=C12)C(=O)O)C1=CC=C(C=C1)C(F)(F)F
For R&D use only. Not for human or animal use.
4-methoxy-2-(4-(trifluoromethyl)phenyl)quinoline-7-carboxylic acid; CAS No.: 2842025-72-1;4-methoxy-2-(4-(trifluoromethyl)phenyl)quinoline-7-carboxylic acid. PROPERTIES: 4-methoxy-2-(4-(trifluoromethyl)phenyl)quinoline-7-carboxylic acid is a substituted quinoline carboxylic acid with a molecular weight of 383.33 g/mol. This white crystalline solid has a melting point ranging from 225-228 C. The quinoline ring bears a methoxy group at position 4 and is fused at position 2 to a phenyl ring substituted with a trifluoromethyl group at its para position. The carboxylic acid function resides at position 7. It exhibits limited water solubility but dissolves in basic aqueous solutions and common organic solvents like DMSO and DMF. Proper storage involves keeping in tightly sealed containers at room temperature, protected from moisture and light. Safety considerations include the trifluoromethyl group's potential to generate toxic fumes upon heating and the carboxylic acid's corrosive nature. Appropriate protective measures should be taken during handling. APPLICATIONS: This compound primarily serves as a building block in the synthesis of anti-inflammatory and anticancer agents, where the trifluoromethylphenyl substituent enhances lipophilicity and the methoxy group modulates electronic properties. In medicinal chemistry, it has been utilized to create COX-2 inhibitors and has shown promise in the development of tyrosine kinase inhibitors for cancer therapy. The quinoline-7-carboxylic acid scaffold also finds application in the preparation of fluorescent dyes and probes, particularly for bioimaging applications where the trifluoromethyl group contributes to solubility and the methoxy group affects fluorescence quantum yield. These applications are supported by research published in Bioorganic & Medicinal Chemistry Letters and the Journal of Fluorine Chemistry.
Reviews
Write Your Own Review