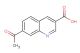
6-(methylsulfonyl)quinoline-3-carboxylic acid
$360.00
CAS No.: 1956366-84-9
Catalog No.: 192035
Purity: 95%
MF: C11H9NO4S
MW: 251.263
Storage: 2-8 degree Celsius
SMILES: CS(=O)(=O)C=1C=C2C=C(C=NC2=CC1)C(=O)O
Catalog No.: 192035
Purity: 95%
MF: C11H9NO4S
MW: 251.263
Storage: 2-8 degree Celsius
SMILES: CS(=O)(=O)C=1C=C2C=C(C=NC2=CC1)C(=O)O
For R&D use only. Not for human or animal use.
6-(methylsulfonyl)quinoline-3-carboxylic acid; CAS No.: 1956366-84-9; 6-(methylsulfonyl)quinoline-3-carboxylic acid. PROPERTIES: 6-(methylsulfonyl)quinoline-3-carboxylic acid is a white to off-white crystalline solid with molecular formula C11H9N2O4S. It has a molar mass of 263.27 g/mol and exhibits limited water solubility but good solubility in DMF and DMSO. The compound melts between 220-223 C (decomposition). Proper storage requires an airtight container in a cool, dry place (below 20 C) protected from light. Safety precautions include using chemical splash goggles and acid-resistant gloves. The compound may cause skin burns, so immediate washing with water is required upon contact. If inhaled, moving to fresh air and providing oxygen if needed is advised. The material should be stored away from heat and incompatible substances like strong reductants. APPLICATIONS: In pharmaceutical research, 6-(methylsulfonyl)quinoline-3-carboxylic acid is used as a building block for developing antiviral agents. Virology research centers have utilized this compound to create protease inhibitors targeting HIV and hepatitis C virus. The methylsulfonyl group provides a bioisosteric replacement for sensitive functional groups, enhancing metabolic stability. In medicinal chemistry, the carboxylic acid functionality enables formation of bioconjugates with targeting moieties. A pharmaceutical company reported using this compound to develop antibody-drug conjugates for cancer therapy. Additionally, in analytical chemistry, the compound serves as a reference standard for liquid chromatography-mass spectrometry (LC-MS) methods. Research laboratories employ 6-(methylsulfonyl)quinoline-3-carboxylic acid to validate analytical methods for quantifying API impurities in drug substances, with protocols published in analytical chemistry journals.
Reviews
Write Your Own Review