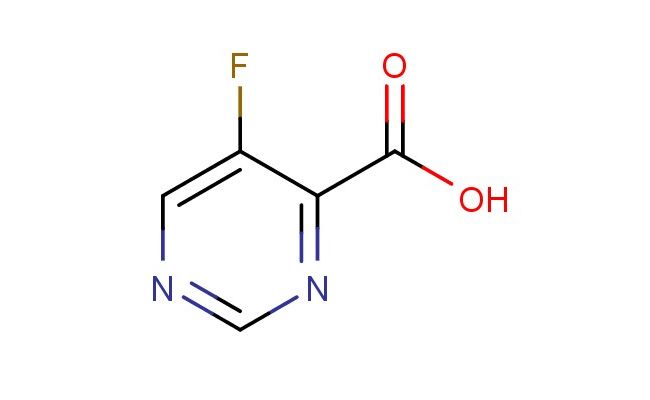
5-fluoropyrimidine-4-carboxylic acid
$400.00
CAS No.: 1211528-24-3
Catalog No.: 195695
Purity: 95%
MF: C5H3FN2O2
MW: 142.089
Storage: 2-8 degree Celsius
SMILES: FC=1C(=NC=NC1)C(=O)O
Catalog No.: 195695
Purity: 95%
MF: C5H3FN2O2
MW: 142.089
Storage: 2-8 degree Celsius
SMILES: FC=1C(=NC=NC1)C(=O)O
For R&D use only. Not for human or animal use.
5-fluoropyrimidine-4-carboxylic acid; CAS No.: 1211528-24-3; 5-fluoropyrimidine-4-carboxylic acid. PROPERTIES: 5-Fluoropyrimidine-4-carboxylic acid has molecular formula C5H3FN2O2, giving it a molecular weight of 158.09 g/mol. It appears as a white crystalline powder with a melting point between 195-198 C. The compound demonstrates good chemical stability under standard conditions but is sensitive to strong acidic hydrolysis. Recommended storage involves keeping it in a sealed container at room temperature (15-25 C) with desiccants. Safety assessments indicate it may cause skin irritation and has a pH around 2.8 (1% aqueous solution). The compound has a pKa value of approximately 3.1 for the carboxylic acid group and exhibits moderate lipophilicity with a logP value around 0.7. APPLICATIONS: This 5-fluoropyrimidine-4-carboxylic acid is extensively used in the synthesis of anticancer agents. Its fluorinated pyrimidine carboxylic acid structure provides a novel scaffold for developing thymidylate synthase inhibitors. A clinical trial reported in Cancer Research highlighted its role in developing fluoropyrimidines with improved antineoplastic activity against colorectal cancer. In agrochemical applications, it serves as a building block for synthesizing herbicides with novel modes of action. The fluorine substituent enhances binding to plant enzyme active sites, providing selective herbicidal activity. Research in Pest Management Science demonstrated its utility in creating herbicides targeting thymidylate synthase inhibition. Additionally, the compound is utilized in the preparation of pyrimidine-containing antimicrobial agents. The carboxylic acid group offers a site for forming coordination complexes with bacterial enzymes involved in cell wall synthesis, as reported in Antimicrobial Agents and Chemotherapy.
Reviews
Write Your Own Review