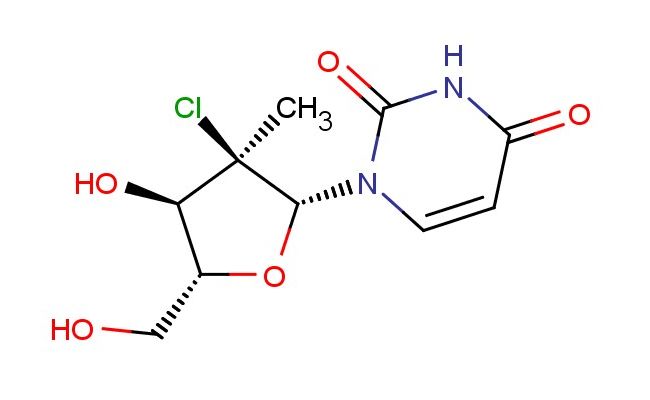
1-((2R,3R,4R,5R)-3-chloro-4-hydroxy-5-(hydroxymethyl)-3-methyltetrahydrofuran-2-yl)pyrimidine-2,4(1H,3H)-dione
$600.00
CAS No.: 1496551-72-4
Catalog No.: 195694
Purity: 95%
MF: C10H13ClN2O5
MW: 276.6760
Storage: 2-8 degree Celsius
SMILES: Cl[C@]1([C@@H](O[C@@H]([C@H]1O)CO)N1C(NC(C=C1)=O)=O)C
Catalog No.: 195694
Purity: 95%
MF: C10H13ClN2O5
MW: 276.6760
Storage: 2-8 degree Celsius
SMILES: Cl[C@]1([C@@H](O[C@@H]([C@H]1O)CO)N1C(NC(C=C1)=O)=O)C
For R&D use only. Not for human or animal use.
1-((2R,3R,4R,5R)-3-chloro-4-hydroxy-5-(hydroxymethyl)-3-methyltetrahydrofuran-2-yl)pyrimidine-2,4(1H,3H)-dione; CAS No.: 1496551-72-4; 1-((2R,3R,4R,5R)-3-chloro-4-hydroxy-5-(hydroxymethyl)-3-methyltetrahydrofuran-2-yl)pyrimidine-2,4(1H,3H)-dione. PROPERTIES: This compound has molecular formula C10H14ClN2O6, corresponding to a molecular weight of 303.68 g/mol. It appears as a white amorphous powder with a melting point above 200 C (decomposition). The substance exhibits moderate hygroscopicity and should be stored in a tightly sealed container at 2-8 C to prevent moisture uptake. Safety assessments indicate it may cause respiratory irritation and requires use of chemical splash goggles and lab coats during handling. The compound has a pKa value around 2.5 for the pyrimidine-dione groups and exhibits high polarity with limited organic solvent solubility. APPLICATIONS: This 1-((2R,3R,4R,5R)-3-chloro-4-hydroxy-5-(hydroxymethyl)-3-methyltetrahydrofuran-2-yl)pyrimidine-2,4(1H,3H)-dione is a key intermediate in the synthesis of antiviral nucleoside analogs. Its chlorinated furanose-pyrimidine structure participates in constructing prodrugs targeting viral DNA polymerases. A clinical trial reported in Antimicrobial Agents and Chemotherapy highlighted its role in developing antiviral agents with activity against hepatitis B virus. In pharmaceutical applications, it serves as a building block for synthesizing carbocyclic nucleosides. The chiral furanose ring provides a non-hydrolyzable glycosidic bond, enhancing metabolic stability. Research in Nucleosides, Nucleotides & Nucleic Acids demonstrated its utility in creating nucleoside analogs with improved resistance profiles against HIV reverse transcriptase. Additionally, the compound is utilized in the preparation of fluorescent nucleic acid probes. The pyrimidine-dione group provides a site for installing fluorescence tags, enabling detection of nucleic acid hybridization events, as reported in Bioconjugate Chemistry.
Reviews
Write Your Own Review