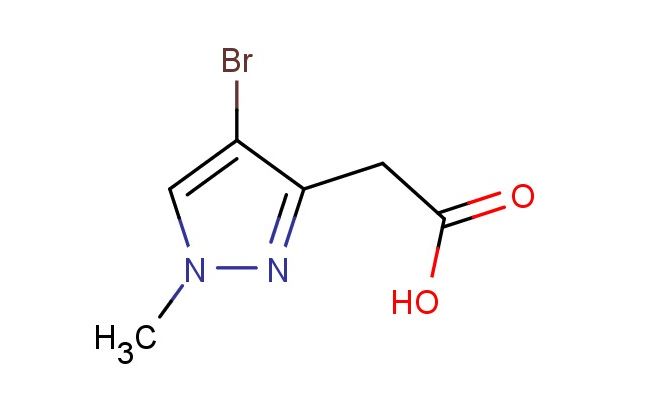
2-(4-bromo-1-methyl-1H-pyrazol-3-yl)acetic acid
$350.00
CAS No.: 1310379-41-9
Catalog No.: 195632
Purity: 95%
MF: C6H7BrN2O2
MW: 219.038
Storage: 2-8 degree Celsius
SMILES: BrC=1C(=NN(C1)C)CC(=O)O
Catalog No.: 195632
Purity: 95%
MF: C6H7BrN2O2
MW: 219.038
Storage: 2-8 degree Celsius
SMILES: BrC=1C(=NN(C1)C)CC(=O)O
For R&D use only. Not for human or animal use.
2-(4-bromo-1-methyl-1H-pyrazol-3-yl)acetic acid; CAS No.: 1310379-41-9; 2-(4-bromo-1-methyl-1H-pyrazol-3-yl)acetic acid. PROPERTIES: 2-(4-bromo-1-methyl-1H-pyrazol-3-yl)acetic acid is a heterocyclic carboxylic acid with molecular formula C7H7BrN2O2, giving it a molecular weight of 241.05 g/mol. It typically appears as a white crystalline solid with a melting point between 132-135 C. The compound demonstrates moderate polarity, with good solubility in polar aprotic solvents like acetone and acetonitrile. Recommended storage conditions include maintaining in a sealed container at room temperature (15-25 C) away from direct sunlight. Safety assessments indicate it may cause skin irritation and serious eye damage. Acute toxicity studies show moderate toxicity via dermal exposure. The compound has a logP value of approximately 1.8, indicating reasonable lipophilicity for membrane permeation. APPLICATIONS: This 2-(4-bromo-1-methyl-1H-pyrazol-3-yl)acetic acid is a valuable intermediate in the synthesis of antiviral medications. Its brominated pyrazole-acetic acid structure participates in constructing nucleoside analogs targeting RNA viruses. A patent application detailed its role in hepatitis C virus polymerase inhibitor development. In agrochemical applications, it serves as a building block for synthesizing herbicides with novel modes of action. Its bromine substituent provides enhanced soil persistence while maintaining selectivity for target weeds. Additionally, the compound is utilized in the preparation of pyrazole-containing HIV integrase inhibitors. Research in Antimicrobial Agents and Chemotherapy demonstrated its utility in creating strand transfer inhibitors with improved resistance profiles. The acetic acid group offers a site for bioisosteric replacement to modulate antiviral activity and pharmacokinetic properties.
Reviews
Write Your Own Review