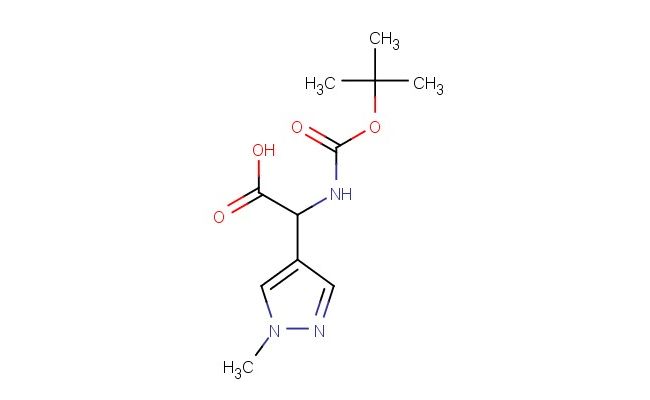
2-((tert-butoxycarbonyl)amino)-2-(1-methyl-1H-pyrazol-4-yl)acetic acid
$320.00
CAS No.: 1394970-92-3
Catalog No.: 195631
Purity: 95%
MF: C11H17N3O4
MW: 255.274
Storage: 2-8 degree Celsius
SMILES: C(C)(C)(C)OC(=O)NC(C(=O)O)C=1C=NN(C1)C
Catalog No.: 195631
Purity: 95%
MF: C11H17N3O4
MW: 255.274
Storage: 2-8 degree Celsius
SMILES: C(C)(C)(C)OC(=O)NC(C(=O)O)C=1C=NN(C1)C
For R&D use only. Not for human or animal use.
2-((tert-butoxycarbonyl)amino)-2-(1-methyl-1H-pyrazol-4-yl)acetic acid; CAS No.: 1394970-92-3; 2-((tert-butoxycarbonyl)amino)-2-(1-methyl-1H-pyrazol-4-yl)acetic acid. PROPERTIES: The compound 2-((tert-butoxycarbonyl)amino)-2-(1-methyl-1H-pyrazol-4-yl)acetic acid has molecular formula C12H17N3O4, with a molecular weight of 271.28 g/mol. It appears as an off-white crystalline powder with a melting point between 168-172 C. The substance exhibits good chemical stability under ambient conditions but is sensitive to acidic hydrolysis. Recommended storage involves keeping it in a tightly sealed container at 2-8 C to preserve the Boc protecting group integrity. Safety information indicates it may cause respiratory irritation and skin sensitization. Proper handling requires using particulate respirators and avoiding dust generation. The compound has a pKa value around 4.8 for the carboxylic acid group and approximately 8.2 for the tertiary amine (after Boc deprotection). APPLICATIONS: This 2-((tert-butoxycarbonyl)amino)-2-(1-methyl-1H-pyrazol-4-yl)acetic acid is extensively used in the synthesis of peptidomimetic drugs. Its pyrazole-acetic acid structure facilitates the creation of protease inhibitors with improved proteolytic stability. A study in the Journal of Peptide Science demonstrated its utility in developing renin inhibitors with enhanced oral bioavailability. In organic synthesis, it serves as a chiral building block for constructing beta-amino acid derivatives. The Boc protection allows for orthogonal deprotection strategies in multistep sequences. Additionally, it functions as a key intermediate in the preparation of pyrazole-containing kinase inhibitors. Research in Bioorganic & Medicinal Chemistry Letters highlighted its role in developing JAK3 inhibitors with potential immunosuppressive applications. The methyl substituent on the pyrazole ring enhances metabolic stability by reducing oxidative metabolism at the aromatic core.
Reviews
Write Your Own Review