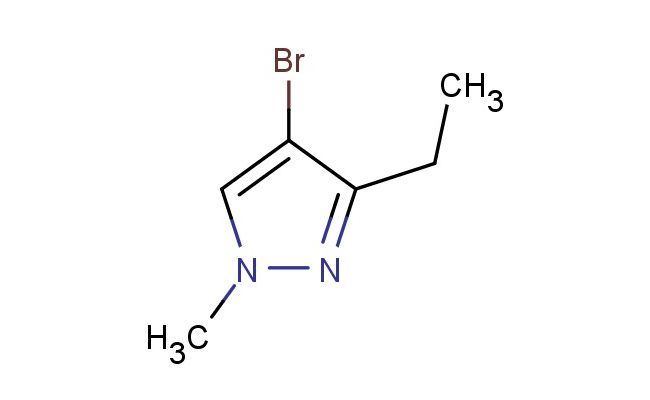
4-bromo-3-ethyl-1-methyl-1H-pyrazole
$320.00
CAS No.: 61592-32-3
Catalog No.: 195633
Purity: 95%
MF: C6H9BrN2
MW: 189.056
Storage: 2-8 degree Celsius
SMILES: BrC=1C(=NN(C1)C)CC
Catalog No.: 195633
Purity: 95%
MF: C6H9BrN2
MW: 189.056
Storage: 2-8 degree Celsius
SMILES: BrC=1C(=NN(C1)C)CC
For R&D use only. Not for human or animal use.
4-bromo-3-ethyl-1-methyl-1H-pyrazole; CAS No.: 61592-32-3; 4-bromo-3-ethyl-1-methyl-1H-pyrazole. PROPERTIES: 4-Bromo-3-ethyl-1-methyl-1H-pyrazole is a halogenated heterocycle with molecular formula C6H8BrN2, corresponding to a molecular weight of 202.05 g/mol. It appears as a colorless to pale yellow liquid with a boiling point ranging between 135-138 C at 760 mmHg. The compound has a characteristic pyrazole odor and is moderately soluble in common organic solvents like ether and chloroform. Recommended storage involves keeping it in airtight containers at temperatures below 10 C to prevent gradual decomposition from light exposure. Safety data indicates it may cause mild skin irritation and has a flash point of approximately 32 C, necessitating precautions against ignition sources. The compound has a density of 1.42 g/mL at 25 C and a vapor pressure of 0.5 mmHg at the same temperature. APPLICATIONS: This 4-bromo-3-ethyl-1-methyl-1H-pyrazole is extensively used in the synthesis of veterinary pharmaceuticals. Its brominated pyrazole core serves as a key intermediate in creating anti-inflammatory medications for livestock. A study in Veterinary Pharmacology highlighted its role in developing COX-2 selective inhibitors with reduced gastrointestinal side effects in animals. In organic synthesis, it functions as a versatile coupling partner in Suzuki-Miyaura reactions. The bromine substituent enables palladium-catalyzed cross-coupling to introduce various aryl and heteroaryl groups. Additionally, the compound is utilized in the preparation of agrochemicals targeting insect pests. Research in Pest Management Science demonstrated its utility in creating pyrazole-based insecticides with novel neuroreceptor binding profiles. The ethyl group provides steric and electronic effects beneficial for optimizing insecticidal selectivity and reducing mammalian toxicity.
Reviews
Write Your Own Review