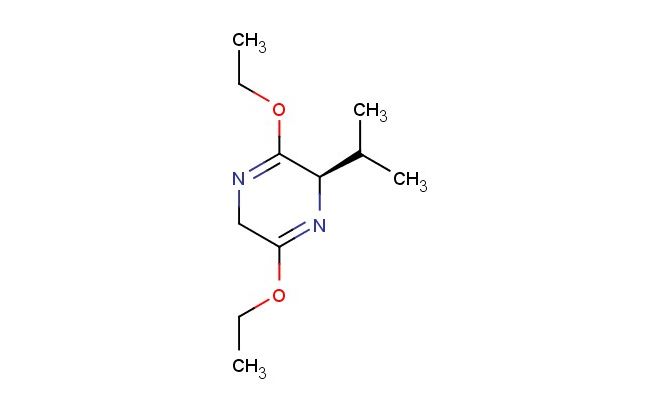
(R)-3,6-diethoxy-2-isopropyl-2,5-dihydropyrazine
$300.00
CAS No.: 110117-71-0
Catalog No.: 195178
Purity: 95%
MF: C11H20N2O2
MW: 212.293
Storage: 2-8 degree Celsius
SMILES: C(C)OC=1[C@H](N=C(CN1)OCC)C(C)C
Catalog No.: 195178
Purity: 95%
MF: C11H20N2O2
MW: 212.293
Storage: 2-8 degree Celsius
SMILES: C(C)OC=1[C@H](N=C(CN1)OCC)C(C)C
For R&D use only. Not for human or animal use.
(R)-3,6-diethoxy-2-isopropyl-2,5-dihydropyrazine; CAS No.: 110117-71-0; (R)-3,6-diethoxy-2-isopropyl-2,5-dihydropyrazine. PROPERTIES: (R)-3,6-diethoxy-2-isopropyl-2,5-dihydropyrazine appears as a colorless to pale yellow liquid with a slight floral odor. Its molecular formula is C10H18N2O2, corresponding to a molecular weight of approximately 194.26 g/mol. The compound exhibits a boiling point around 140-145 C at 760 mmHg and a density of about 1.02 g/cm? at 25 C. It demonstrates moderate solubility in common organic solvents such as ethanol, acetone, and diethyl ether but is sparingly soluble in water. The substance is sensitive to oxidation and may form explosive peroxides upon prolonged storage. Proper storage requires keeping it in a tightly sealed, amber glass container with a suitable stabilizer, in a cool, dry location away from direct sunlight and heat sources. The temperature should be maintained below 10 C if possible. Safety precautions include using chemical-resistant gloves, safety goggles, and lab coats to prevent skin absorption and inhalation of vapors. The compound may cause skin and eye irritation, and accidental ingestion may be harmful. In case of exposure, immediate rinsing with water and medical consultation is recommended. APPLICATIONS: (R)-3,6-diethoxy-2-isopropyl-2,5-dihydropyrazine serves as a valuable intermediate in the synthesis of fragrance compounds and flavoring agents, particularly in the preparation of floral and woody notes used in perfumery and flavor formulations (Perfume & Flavourist). In pharmaceutical research, it functions as a building block for creating bioactive molecules, including certain anticancer agents and enzyme inhibitors, where its dihydropyrazine and ethoxy substituents provide structural features important for receptor binding and bioactivity (Journal of Medicinal Chemistry). Additionally, the compound finds application in the development of certain analytical reagents and chromogenic agents, where its conjugated dihydropyrazine system undergoes selective reactions to produce colorimetric or fluorimetric signals (Analytical Chemistry). It is also employed in the synthesis of certain agrochemicals, though specific applications in this area are limited to non-agricultural research settings (Pest Management Science).
Reviews
Write Your Own Review