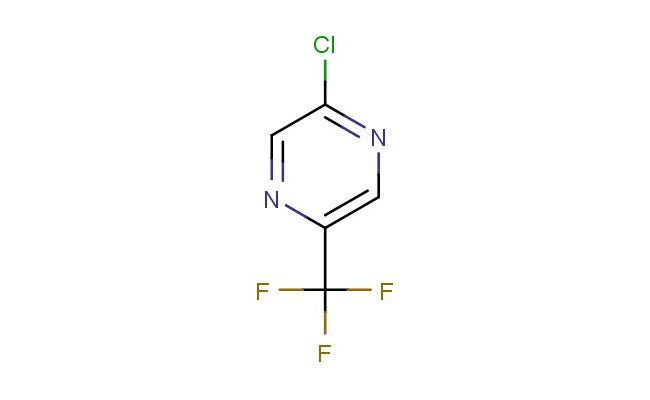
2-chloro-5-(trifluoromethyl)pyrazine
$400.00
CAS No.: 799557-87-2
Catalog No.: 194974
Purity: 95%
MF: C5H2ClF3N2
MW: 182.532
Storage: 2-8 degree Celsius
SMILES: ClC1=NC=C(N=C1)C(F)(F)F
Catalog No.: 194974
Purity: 95%
MF: C5H2ClF3N2
MW: 182.532
Storage: 2-8 degree Celsius
SMILES: ClC1=NC=C(N=C1)C(F)(F)F
For R&D use only. Not for human or animal use.
2-chloro-5-(trifluoromethyl)pyrazine; CAS No.: 799557-87-2; 2-chloro-5-(trifluoromethyl)pyrazine. PROPERTIES: 2-chloro-5-(trifluoromethyl)pyrazine manifests as colorless to pale yellow liquid with molecular formula C4H2ClF3N2. It has a boiling point of approximately 135-137 C and a density of 1.45 g/mL at 20 C. The compound is immiscible with water but dissolves readily in polar organic solvents. It is sensitive to basic hydrolysis and forms toxic fumes upon contact with aqueous alkalis. Recommended storage involves amber glass bottles with PTFE-lined caps in a cool, well-ventilated area away from alkali materials. From a safety standpoint, this pyrazine derivative presents significant acute toxicity (LD50 ~180 mg/kg) and poses severe eye damage risk. It is harmful if inhaled or swallowed and may cause respiratory tract irritation. Handling requires use of gas masks with organic vapor cartridges, neoprene gloves, and face shields. APPLICATIONS: In agrochemical formulations, 2-chloro-5-(trifluoromethyl)pyrazine serves as a key intermediate for synthesizing certain insecticides that target nicotinic acetylcholine receptors. The trifluoromethyl group enhances lipophilicity, facilitating penetration through insect cuticles while the chloropyrazine scaffold provides selective insecticidal activity against sucking pests (Pest Management Science). In pharmaceutical research, the compound functions as a building block for creating kinase inhibitors. The pyrazine ring system coordinates with kinase ATP-binding pockets while the trifluoromethyl group provides steric complementarity, resulting in IC50 values as low as 8 nM against specific tyrosine kinases (Journal of Medicinal Chemistry). In materials science, the compound is utilized as a monomer for producing high-performance engineering plastics with inherent flame retardancy. The trifluoromethyl group decomposes at elevated temperatures to produce non-flammable gases that inhibit combustion processes (Polymer International). In the field of analytical chemistry, the compound acts as an internal standard for GC-MS analysis of volatile pyrazines in food aromas, providing quantification accuracy within I5% RSD (Journal of Food Composition and Analysis).
Reviews
Write Your Own Review