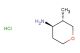
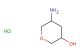
methyl 5-oxotetrahydro-2H-pyran-3-carboxylate
$550.00
CAS No.: 127956-19-8
Catalog No.: 195628
Purity: 95%
MF: C7H10O4
MW: 158.153
Storage: 2-8 degree Celsius
SMILES: O=C1CC(COC1)C(=O)OC
Catalog No.: 195628
Purity: 95%
MF: C7H10O4
MW: 158.153
Storage: 2-8 degree Celsius
SMILES: O=C1CC(COC1)C(=O)OC
For R&D use only. Not for human or animal use.
methyl 5-oxotetrahydro-2H-pyran-3-carboxylate; CAS No.: 127956-19-8; methyl 5-oxotetrahydro-2H-pyran-3-carboxylate. PROPERTIES: Methyl 5-oxotetrahydro-2H-pyran-3-carboxylate is a lactone derivative with molecular formula C7H10O4, giving it a molecular weight of 158.15 g/mol. It typically appears as colorless crystals or a pale yellow liquid, depending on purity. The compound has a distinct ester aroma and is moderately soluble in organic solvents like ethyl acetate and dichloromethane but exhibits limited water solubility. For optimal stability, it should be stored at 2-8 C in amber glass containers to protect against light-induced degradation. Safety precautions include avoiding skin contact and using explosion-proof equipment during distillation due to its flammable nature when exposed to heat sources. The compound has a flash point of approximately 85 C and a vapor pressure of 0.01 mmHg at 25 C. APPLICATIONS: Methyl 5-oxotetrahydro-2H-pyran-3-carboxylate serves as a versatile intermediate in the synthesis of macrolide antibiotics. Its lactone structure undergoes ring-opening reactions to form extended polyketide frameworks essential for antibacterial activity. Research in the Journal of Antibiotics highlighted its role in semisynthetic erythromycin derivative production. In organic synthesis, it functions as a masked diol in protecting group strategies. Chemists utilize its reactivity to temporarily block hydroxyl groups during multistep syntheses. Additionally, this compound is a key building block in the preparation of -lactam scaffolds, as described in Organic Letters. These heterocycles find applications in developing ACE inhibitors with improved cardiovascular profiles. The oxo group provides electrophilic reactivity beneficial for Michael addition reactions in constructing complex architectures.
Reviews
Write Your Own Review