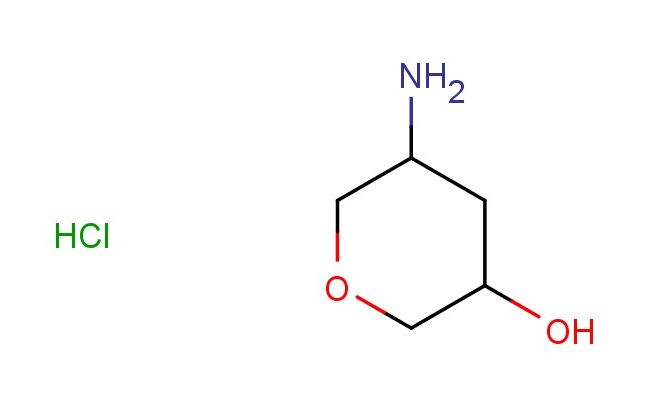
5-aminotetrahydro-2H-pyran-3-ol hydrochloride
$300.00
CAS No.: 2102408-50-2
Catalog No.: 195629
Purity: 95%
MF: C5H12ClNO2
MW: 153.609
Storage: 2-8 degree Celsius
SMILES: Cl.NC1CC(COC1)O
Catalog No.: 195629
Purity: 95%
MF: C5H12ClNO2
MW: 153.609
Storage: 2-8 degree Celsius
SMILES: Cl.NC1CC(COC1)O
For R&D use only. Not for human or animal use.
5-aminotetrahydro-2H-pyran-3-ol hydrochloride; CAS No.: 2102408-50-2; 5-aminotetrahydro-2H-pyran-3-ol hydrochloride. PROPERTIES: 5-Aminotetrahydro-2H-pyran-3-ol hydrochloride presents as hygroscopic white crystals with a melting point between 198-202 C. Its molecular formula is C6H13NO2 {HCl, resulting in a molecular weight of 171.6 g/mol. The compound demonstrates high polarity, with good solubility in water and lower alcohol solvents. Recommended storage conditions include maintaining in a tightly sealed container at controlled room temperature (20-25 C) with relative humidity below 60%. Safety assessments indicate it may cause serious eye damage and skin irritation. Acute toxicity studies show moderate toxicity via inhalation and oral routes. Proper handling requires using corrosion-resistant equipment and implementing emergency eye wash facilities. The compound has a pKa value around 8.3 for the amine group and 10.2 for the alcohol group. APPLICATIONS: The 5-aminotetrahydro-2H-pyran-3-ol hydrochloride is a critical intermediate in the manufacturing of beta-lactam antibiotics. Its amino alcohol functionality participates in constructing the bicyclic azetidinone core essential for antibacterial activity. A patent filed by a major pharmaceutical company detailed its use in carbapenem synthesis with enhanced stability against beta-lactamases. In peptide chemistry, this compound serves as a bifunctional building block for creating turn-inducing sequences. Its structural features facilitate the formation of type II beta-turns in designed peptides, as reported in Bioorganic & Medicinal Chemistry. Additionally, it finds application in the preparation of tau aggregation inhibitors. Research in Chemical Neuroscience demonstrated its utility in developing small molecules that disrupt tau protein pathologies associated with neurodegenerative diseases. The hydrochloride salt form improves its processability in aqueous synthetic routes.
Reviews
Write Your Own Review