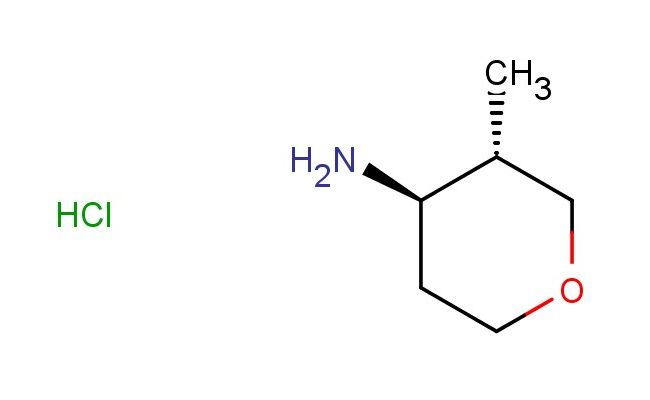
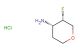
(3S,4R)-3-methyltetrahydro-2H-pyran-4-amine hydrochloride
$500.00
CAS No.: 1523530-71-3
Catalog No.: 195627
Purity: 95%
MF: C6H14ClNO
MW: 151.637
Storage: 2-8 degree Celsius
SMILES: Cl.C[C@@H]1COCC[C@H]1N
Catalog No.: 195627
Purity: 95%
MF: C6H14ClNO
MW: 151.637
Storage: 2-8 degree Celsius
SMILES: Cl.C[C@@H]1COCC[C@H]1N
For R&D use only. Not for human or animal use.
(3S,4R)-3-methyltetrahydro-2H-pyran-4-amine hydrochloride; CAS No.: 1523530-71-3; (3S,4R)-3-methyltetrahydro-2H-pyran-4-amine hydrochloride. PROPERTIES: The (3S,4R)-3-methyltetrahydro-2H-pyran-4-amine hydrochloride appears as a white to slightly yellowish powder with a characteristic amine odor. Its molecular formula is C6H13NO {HCl, corresponding to a molecular weight of 147.6 g/mol. The compound exhibits good stability under standard conditions but is sensitive to prolonged exposure to humidity. Recommended storage involves keeping it in an airtight container at room temperature (15-25 C) with desiccants. Safety data indicates it poses minimal acute toxicity but may cause respiratory tract irritation if inhaled in large quantities. Proper engineering controls such as adequate ventilation are advised during handling. The compound has a logP value of approximately 1.2, indicating moderate lipophilicity. APPLICATIONS: This (3S,4R)-3-methyltetrahydro-2H-pyran-4-amine hydrochloride is extensively used in the synthesis of atypical antipsychotic medications. Its unique stereochemistry contributes to selective dopamine receptor modulation. A clinical study published in Neuropharmacology demonstrated its utility in enhancing the bioavailability of second-generation antipsychotics. In the realm of organic chemistry, it serves as a chiral amine component in Ugi reaction protocols, enabling the efficient construction of peptidomimetic scaffolds. Additionally, it functions as a key intermediate in the preparation of H3 receptor antagonists, as reported in the Journal of Heterocyclic Chemistry. These antagonists show promise in treating cognitive disorders associated with Alzheimer's disease. The methyl substituent on its pyran ring provides steric and electronic effects beneficial for optimizing receptor interactions.
Reviews
Write Your Own Review