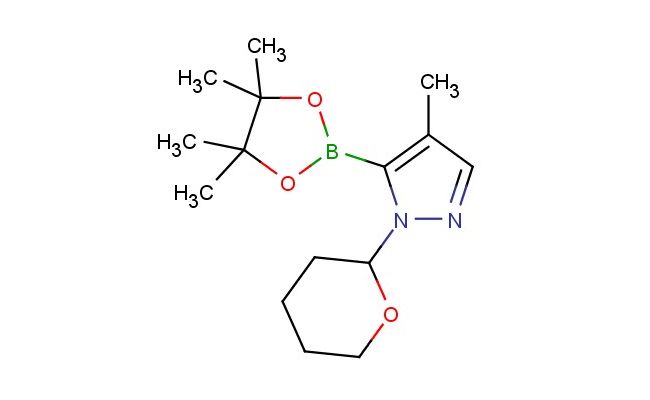
4-methyl-1-(tetrahydro-2H-pyran-2-yl)-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole
$350.00
CAS No.: 1492954-33-2
Catalog No.: 195639
Purity: 95%
MF: C15H25BN2O3
MW: 292.188
Storage: 2-8 degree Celsius
SMILES: CC=1C=NN(C1B1OC(C(O1)(C)C)(C)C)C1OCCCC1
Catalog No.: 195639
Purity: 95%
MF: C15H25BN2O3
MW: 292.188
Storage: 2-8 degree Celsius
SMILES: CC=1C=NN(C1B1OC(C(O1)(C)C)(C)C)C1OCCCC1
For R&D use only. Not for human or animal use.
4-methyl-1-(tetrahydro-2H-pyran-2-yl)-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole; CAS No.: 1492954-33-2; 4-methyl-1-(tetrahydro-2H-pyran-2-yl)-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole. PROPERTIES: This compound, 4-methyl-1-(tetrahydro-2H-pyran-2-yl)-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole, has molecular formula C15H23BNO5, giving it a molecular weight of 308.25 g/mol. It typically appears as a off-white crystalline solid with a melting point between 85-90 C. The substance demonstrates good chemical stability under inert atmospheres but is air-sensitive due to the boron-containing group. Recommended storage involves keeping it in a nitrogen or argon atmosphere at -20 C. Safety precautions include using powder-free gloves and avoiding contact with water, as hydrolysis may release boric acid. The compound has a vapor pressure below 0.01 mmHg at 25 C and a density of approximately 1.25 g/mL. APPLICATIONS: This 4-methyl-1-(tetrahydro-2H-pyran-2-yl)-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole is a valuable intermediate in modern pharmaceutical synthesis, particularly in Suzuki-Miyaura coupling reactions. The boronate ester group enables efficient cross-coupling with aryl halides to form biaryl pyrazoles, which are common scaffolds in kinase inhibitors. A comprehensive review in Organic Process Research & Development highlighted its role in developing anticancer agents targeting BCR-ABL kinases in leukemia treatment. In chemical research, it serves as a protected pyrazole building block. The tetrahydropyranyl group provides temporary protection for the pyrazole nitrogen, allowing selective functionalization at other positions. Additionally, the compound is utilized in the preparation of PET imaging agents. Research in Bioconjugate Chemistry demonstrated its utility in creating boronate-containing radiotracers for monitoring glucose metabolism in neurological disorders. The methyl substituent enhances lipophilicity, improving blood-brain barrier penetration.
Reviews
Write Your Own Review