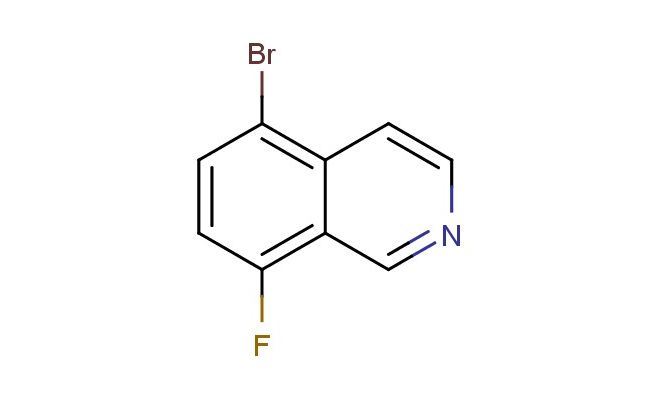
5-bromo-8-fluoroisoquinoline
$200.00
CAS No.: 679433-94-4
Catalog No.: 191962
Purity: 95%
MF: C9H5BrFN
MW: 226.048
Storage: 2-8 degree Celsius
SMILES: BrC1=C2C=CN=CC2=C(C=C1)F
Catalog No.: 191962
Purity: 95%
MF: C9H5BrFN
MW: 226.048
Storage: 2-8 degree Celsius
SMILES: BrC1=C2C=CN=CC2=C(C=C1)F
5-bromo-8-fluoroisoquinoline; CAS No.: 679433-94-4; 5-bromo-8-fluoroisoquinoline. PROPERTIES: 5-bromo-8-fluoroisoquinoline (CAS No.: 679433-94-4) presents as a white to off-white crystalline powder with a melting point between 120-123 C. The compound features a bromine atom at position 5 and a fluorine atom at position 8 of the isoquinoline ring system. These substituents impart significant electron-withdrawing effects and lipophilicity to the molecule. Solubility characteristics reveal moderate dissolvability in polar organic solvents like DMSO and DMF, while being sparingly soluble in water due to the hydrophobic aromatic system. The substance is moisture-sensitive and prone to hydrolysis under basic conditions, necessitating storage in a tightly sealed container under nitrogen atmosphere at controlled room temperature (15-25 C). Safety precautions include using P295 respiratory protection, chemical-resistant gloves, and eye protection due to potential skin irritation and eye damage. The compound has a moderate vapor pressure and forms flammable mixtures with air above 40 C. APPLICATIONS: 5-bromo-8-fluoroisoquinoline serves as a versatile intermediate in developing kinase inhibitors for cancer therapy as documented in oncology research. Its brominated and fluorinated isoquinoline core enables the synthesis of antiviral agents targeting RNA viruses as reported in virology literature. Additionally, 679433-94-4 contributes to the development of fluorescent probes for detecting specific protein targets in cellular environments, with several studies highlighting its utility in creating pH-sensitive indicators for monitoring lysosomal conditions during drug delivery. The compound also functions as a building block for agrochemical intermediates used in developing herbicides with novel mechanisms of action, as described in agricultural chemistry literature.
Reviews
Write Your Own Review