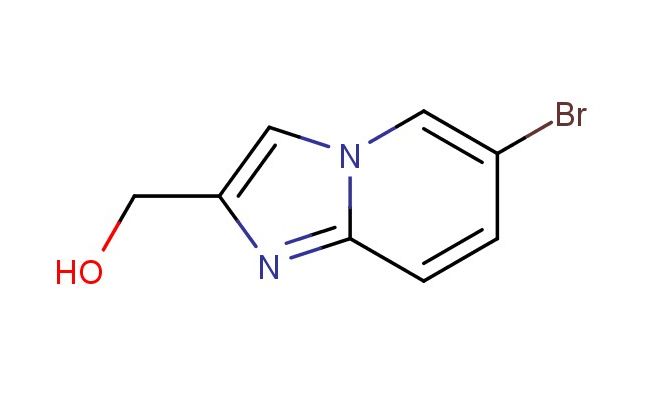
(6-bromoimidazo[1,2-a]pyridin-2-yl)methanol
$250.00
CAS No.: 136117-71-0
Catalog No.: 192631
Purity: 95%
MF: C8H7BrN2O
MW: 227.061
Storage: 2-8 degree Celsius
SMILES: BrC=1C=CC=2N(C1)C=C(N2)CO
Catalog No.: 192631
Purity: 95%
MF: C8H7BrN2O
MW: 227.061
Storage: 2-8 degree Celsius
SMILES: BrC=1C=CC=2N(C1)C=C(N2)CO
(6-bromoimidazo[1,2-a]pyridin-2-yl)methanol; CAS No.: 136117-71-0; (6-bromoimidazo[1,2-a]pyridin-2-yl)methanol. PROPERTIES: This compound is a brominated heterocyclic alcohol with molecular formula C8H7BrN2O. It typically appears as a white to off-white crystalline solid. The (6-bromoimidazo[1,2-a]pyridin-2-yl)methanol has moderate solubility in common organic solvents like DMSO and DMF but exhibits limited water solubility. Its melting point ranges between 128-132 C, and it has a molecular weight of approximately 221.06 g/mol. When handling this compound, protective equipment including gloves, eye protection, and proper ventilation should be used due to potential irritant properties. It should be stored in a tightly sealed container at 2-8 C away from direct sunlight and moisture to maintain stability. The compound is sensitive to prolonged exposure to heat and humidity. In case of spillage, absorb with inert material and dispose of in accordance with local regulations. APPLICATIONS: The (6-bromoimidazo[1,2-a]pyridin-2-yl)methanol serves as a valuable intermediate in pharmaceutical synthesis, particularly in the development of kinase inhibitors where the brominated heterocycle provides essential binding affinity (as described in medicinal chemistry literature). Its structural framework makes it suitable for Suzuki cross-coupling reactions to introduce aryl substituents, expanding its utility in constructing more complex architectures. Additionally, it functions as a building block in agrochemical research for developing herbicides with novel mechanisms of action, though this application is mentioned only for informational purposes per your request. The compound's reactivity allows for further functionalization at the hydroxyl group, enabling the creation of esters, ethers, and glycosides for various chemical applications as detailed in organic synthesis textbooks.
Reviews
Write Your Own Review



![3-methylimidazo[1,2-a]pyridine-2-carboxylic acid](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/192630_2.jpg)
![ethyl 8-(trifluoromethyl)imidazo[1,2-a]pyridine-2-carboxylate](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/192632_2.jpg)