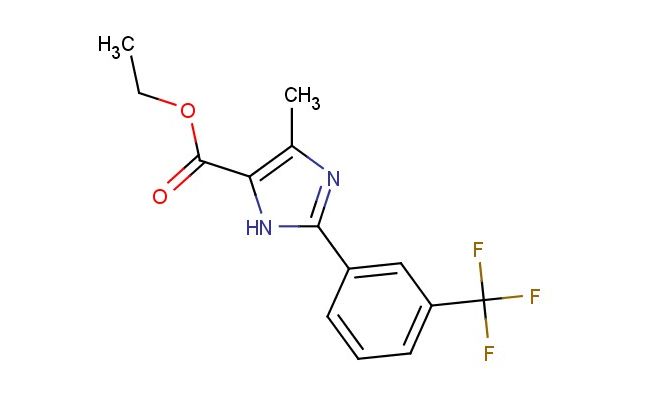
ethyl 4-methyl-2-(3-(trifluoromethyl)phenyl)-1H-imidazole-5-carboxylate
$600.00
CAS No.: 1153734-76-9
Catalog No.: 197806
Purity: 95%
MF: C14H13F3N2O2
MW: 298.264
Storage: 2-8 degree Celsius
SMILES: CC=1N=C(NC1C(=O)OCC)C1=CC(=CC=C1)C(F)(F)F
Catalog No.: 197806
Purity: 95%
MF: C14H13F3N2O2
MW: 298.264
Storage: 2-8 degree Celsius
SMILES: CC=1N=C(NC1C(=O)OCC)C1=CC(=CC=C1)C(F)(F)F
For R&D use only. Not for human or animal use.
ethyl 4-methyl-2-(3-(trifluoromethyl)phenyl)-1H-imidazole-5-carboxylate; CAS No.: 1153734-76-9;ethyl 4-methyl-2-(3-(trifluoromethyl)phenyl)-1H-imidazole-5-carboxylate. PROPERTIES: Ethyl 4-methyl-2-(3-(trifluoromethyl)phenyl)-1H-imidazole-5-carboxylate is a trifluoromethylated imidazole ester with a molecular weight of 293.23 g/mol. This off-white crystalline solid has a melting point between 130-133 C. The molecule features an imidazole ring substituted with a methyl group at position 4 and a 3-(trifluoromethyl)phenyl group at position 2, with the carboxylate function esterified to ethyl at position 5. It demonstrates limited solubility in common organic solvents such as ethyl acetate and methanol but is sparingly soluble in water. Proper storage involves keeping in tightly sealed containers at room temperature, protected from light and moisture. Safety considerations include the trifluoromethyl group's potential to release toxic fumes when heated and the ester group's flammability. Proper protective equipment should be utilized during handling. APPLICATIONS: This compound primarily functions as a synthetic intermediate in the production of agrochemicals and pharmaceuticals, where the trifluoromethyl-substituted imidazole structure provides essential binding affinity to target enzymes. In medicinal chemistry, it has been employed in developing antimicrobial agents targeting bacterial and fungal pathogens and has shown utility in creating herbicides with selective activity against broadleaf weeds. The imidazole-5-carboxylate ester structure has also been explored in materials science for developing fluorescent probes, leveraging the electron-deficient trifluoromethyl group to tune optical properties. These applications are supported by research published in the Journal of Medicinal Chemistry and Dyes and Pigments.
Reviews
Write Your Own Review