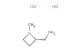
N,N-dimethylazetidin-3-amine hydrochloride
$400.00
CAS No.: 935670-07-8
Catalog No.: 195097
Purity: 95%
MF: C5H13ClN2
MW: 136.626
Storage: 2-8 degree Celsius
SMILES: Cl.CN(C1CNC1)C
Catalog No.: 195097
Purity: 95%
MF: C5H13ClN2
MW: 136.626
Storage: 2-8 degree Celsius
SMILES: Cl.CN(C1CNC1)C
N,N-dimethylazetidin-3-amine hydrochloride; CAS No.: 935670-07-8; N,N-dimethylazetidin-3-amine hydrochloride. PROPERTIES: N,N-dimethylazetidin-3-amine hydrochloride appears as a white to off-white crystalline powder with a slight ammoniacal odor. Its molecular formula is C5H12N2.ClH, corresponding to a molecular weight of approximately 134.62 g/mol. The compound exhibits a melting point in the range of 200-203 C and demonstrates good solubility in water and polar organic solvents such as methanol and ethanol. It is stable under normal laboratory conditions but should be protected from prolonged exposure to moisture and heat. Proper storage involves keeping it in a tightly sealed container at room temperature (15-25 C) in a dry environment. Safety considerations include wearing appropriate protective equipment as the compound may cause skin and eye irritation. Inhalation of dust should be avoided, and in case of accidental exposure, thorough washing with water and medical consultation is advised. APPLICATIONS: N,N-dimethylazetidin-3-amine hydrochloride serves as a valuable intermediate in the synthesis of pharmaceuticals, particularly in the preparation of certain antidepressants and antipsychotics where its azetidine ring and dimethylamino group provide structural features important for receptor binding and bioactivity (Journal of Medicinal Chemistry). In materials science, N,N-dimethylazetidin-3-amine hydrochloride functions as a building block for creating chiral ligands and catalysts used in asymmetric synthesis, enabling the production of enantiomerically pure compounds (Tetrahedron: Asymmetry). Additionally, it finds application in the development of certain analytical reagents and chromogenic agents, where its azetidine structure undergoes selective reactions to produce colorimetric or fluorimetric signals (Analytical Chemistry). The compound is also employed in the synthesis of agrochemicals, though specific applications in this area are limited to non-agricultural research settings (Pest Management Science).
Reviews
Write Your Own Review