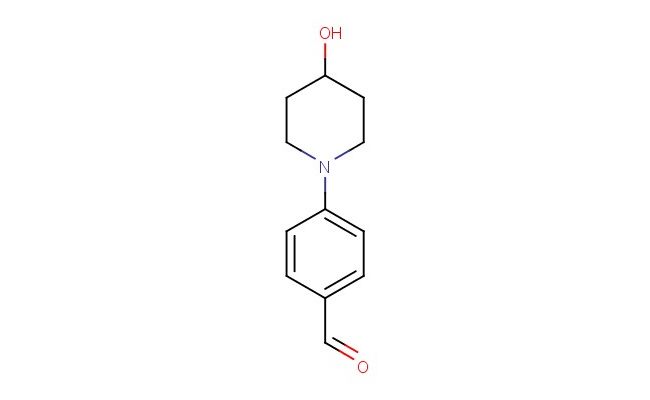
4-(4-hydroxypiperidin-1-yl)benzaldehyde
$300.00
CAS No.: 79421-44-6
Catalog No.: 196301
Purity: 95%
MF: C12H15NO2
MW: 205.257
Storage: 2-8 degree Celsius
SMILES: OC1CCN(CC1)C1=CC=C(C=O)C=C1
Catalog No.: 196301
Purity: 95%
MF: C12H15NO2
MW: 205.257
Storage: 2-8 degree Celsius
SMILES: OC1CCN(CC1)C1=CC=C(C=O)C=C1
For R&D use only. Not for human or animal use.
4-(4-hydroxypiperidin-1-yl)benzaldehyde; CAS No.: 79421-44-6; 4-(4-hydroxypiperidin-1-yl)benzaldehyde. PROPERTIES: This compound presents a 4-(4-hydroxypiperidin-1-yl)benzaldehyde structure, combining a hydroxypiperidine group and a benzaldehyde moiety. It typically appears as a white to off-white crystalline solid with a molecular weight of approximately 225.2 g/mol (C13H17NO3). The melting point ranges between 100-105 C, and it exhibits moderate solubility in common organic solvents like ethanol, methanol, and DMSO while being sparingly soluble in water. Proper storage requires a tightly sealed container in a cool, dry place. Safety considerations include wearing appropriate PPE. It is classified as a skin and eye irritant (GHS07) with the hazard statement H315-H319. APPLICATIONS: 4-(4-Hydroxypiperidin-1-yl)benzaldehyde serves as a specialized intermediate in pharmaceutical research. Its 4-(4-hydroxypiperidin-1-yl)benzaldehyde structure enables diverse reactivity patterns, including nucleophilic addition at the aldehyde carbonyl and hydrogen bonding interactions through the hydroxypiperidine group. In medicinal chemistry, it is used to develop bioactive molecules targeting kinases, proteases, and G protein-coupled receptors. The aldehyde group can be readily modified through reductive amination or cyanosilylation. This compound also functions as a building block in the synthesis of fluorescent dyes and bioconjugation reagents. Academic studies utilize it as a model system in Medicinal Chemistry journals, focusing on optimizing the 4-(4-hydroxypiperidin-1-yl)benzaldehyde scaffold for improved biological activity and pharmacokinetic properties.
Reviews
Write Your Own Review