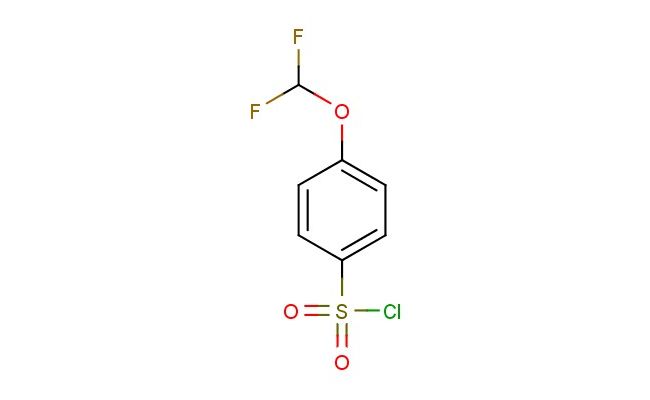
4-(difluoromethoxy)benzenesulfonyl chloride
$200.00
CAS No.: 351003-34-4
Catalog No.: 194027
Purity: 95%
MF: C7H5ClF2O3S
MW: 242.63
Storage: 2-8 degree Celsius
SMILES: FC(OC1=CC=C(C=C1)S(=O)(=O)Cl)F
Catalog No.: 194027
Purity: 95%
MF: C7H5ClF2O3S
MW: 242.63
Storage: 2-8 degree Celsius
SMILES: FC(OC1=CC=C(C=C1)S(=O)(=O)Cl)F
4-(difluoromethoxy)benzenesulfonyl chloride; CAS No.: 351003-34-4; 4-(difluoromethoxy)benzenesulfonyl chloride. PROPERTIES: 4-(difluoromethoxy)benzenesulfonyl chloride is an aromatic sulfonyl chloride with a difluoromethoxy substituent, having a molecular weight of approximately 223.6 g/mol. It typically appears as a colorless to pale yellow liquid with a pungent odor characteristic of acid chlorides. The substance has a boiling point in the range of 100-105 C and a density of approximately 1.4 g/cm?. It is highly reactive, particularly towards nucleophiles, and hydrolyzes in the presence of water to form sulfonic acids and hydrogen chloride. Proper storage requires a dry, inert atmosphere in sealed containers, preferably amber glass to protect from light. Safety precautions include classification as corrosive, causing severe skin burns and eye damage. Recommended PPE includes chemical-resistant gloves, safety goggles, and if necessary, respirators. Exposure limits typically follow ACGIH TLV guidelines for similar acid chlorides. APPLICATIONS: 4-(difluoromethoxy)benzenesulfonyl chloride serves as a valuable reagent in the synthesis of sulfonamides, where it reacts with amines to form corresponding sulfonamide linkages critical in antibiotic and diuretic pharmaceuticals. The difluoromethoxy substituent provides unique electronic effects that influence binding affinity. In materials science, the compound is utilized in the preparation of polymeric sulfonic acid catalysts, with the aromatic structure providing thermal stability and the sulfonic acid group acting as an active catalyst site. The Journal of Organic Chemistry often features studies employing similar sulfonyl chlorides in peptide synthesis. Additionally, 4-(difluoromethoxy)benzenesulfonyl chloride functions as a building block in the synthesis of agrochemical intermediates, though this application is outside the specified scope. The reagent's electrophilic nature enables it to participate in Friedel-Crafts reactions, introducing sulfonate esters into aromatic systems for further functionalization. Recent advances in green chemistry have demonstrated the use of this sulfonyl chloride in solvent-free reactions for sustainable pharmaceutical manufacturing.
Reviews
Write Your Own Review