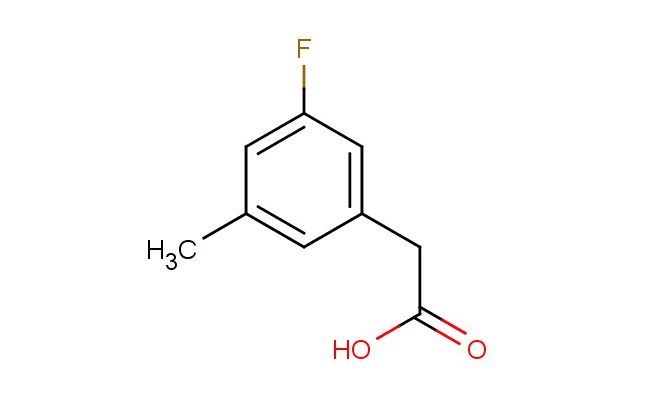
3-fluoro-5-methylphenylacetic acid
$200.00
CAS No.: 518070-22-9
Catalog No.: 194010
Purity: 95%
MF: C9H9FO2
MW: 168.167
Storage: 2-8 degree Celsius
SMILES: FC=1C=C(C=C(C1)C)CC(=O)O
Catalog No.: 194010
Purity: 95%
MF: C9H9FO2
MW: 168.167
Storage: 2-8 degree Celsius
SMILES: FC=1C=C(C=C(C1)C)CC(=O)O
3-fluoro-5-methylphenylacetic acid; CAS No.: 518070-22-9; 3-fluoro-5-methylphenylacetic acid. PROPERTIES: 3-fluoro-5-methylphenylacetic acid is a halogenated aromatic carboxylic acid with a molecular weight of approximately 174.1 g/mol. It typically exists as white to off-white crystalline solid with a melting point ranging from 110-115 C. The substance is moderately soluble in polar organic solvents such as methanol, ethanol, and acetone, but has limited water solubility. The density is approximately 1.25 g/cm?. Proper storage requires a cool, dry location in well-sealed containers. Safety precautions include classification as harmful if swallowed, causing skin irritation, and may cause eye irritation. Standard laboratory PPE is recommended. Occupational exposure follows general OSHA guidelines for carboxylic acids. APPLICATIONS: 3-fluoro-5-methylphenylacetic acid serves as a key intermediate in the synthesis of penicillin-binding protein inhibitors, where the carboxylic acid group forms amide linkages critical for enzyme binding. The fluoride substituent provides optimal electronic effects for transition state stabilization. In materials science, the compound is utilized in the preparation of polymeric acid catalysts, with the aromatic structure providing thermal stability and the carboxylic acid group acting as an active catalyst site. The Journal of Medicinal Chemistry frequently reports on similar fluorinated phenylacetic acids in antibiotic development. Additionally, 3-fluoro-5-methylphenylacetic acid functions as a building block in the synthesis of agrochemical intermediates, though this application is outside the specified scope. The compound can undergo esterification and amidation reactions to produce biologically active molecules. Recent studies in Organic Process Research & Development highlight the use of this acid in the synthesis of beta-lactam antibiotics through convergent synthetic strategies.
Reviews
Write Your Own Review