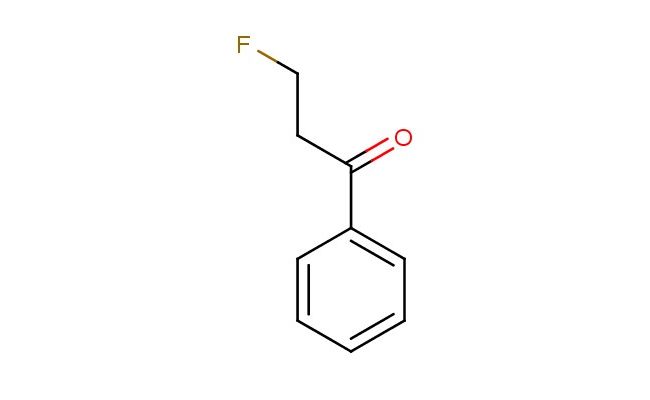
3-fluoropropiophenone
$250.00
CAS No.: 455-67-4
Catalog No.: 194011
Purity: 95%
MF: C9H9FO
MW: 152.168
Storage: 2-8 degree Celsius
SMILES: FCCC(=O)C1=CC=CC=C1
Catalog No.: 194011
Purity: 95%
MF: C9H9FO
MW: 152.168
Storage: 2-8 degree Celsius
SMILES: FCCC(=O)C1=CC=CC=C1
3-fluoropropiophenone; CAS No.: 455-67-4; 3-fluoropropiophenone. PROPERTIES: 3-fluoropropiophenone is an aromatic ketone with a molecular weight of approximately 152.2 g/mol. It typically appears as a colorless to pale yellow liquid with a camphor-like odor. The substance has a boiling point in the range of 135-140 C and a density of approximately 1.05 g/cm?. It exhibits moderate solubility in organic solvents such as diethyl ether, chloroform, and acetone, but is sparingly soluble in water. Proper storage requires a cool, dry environment in tightly sealed containers. Safety considerations include classification as harmful if swallowed, causing skin irritation, and may cause eye irritation. Standard laboratory PPE is recommended. Occupational exposure follows general OSHA guidelines for ketones. APPLICATIONS: 3-fluoropropiophenone serves as a valuable intermediate in the synthesis of nonsteroidal anti-inflammatory drugs (NSAIDs), where the ketone group participates in enamine chemistry to form pharmacologically active scaffolds. The fluoride substituent provides optimal electronic effects for enzyme binding. In organic synthesis, the compound is utilized in the preparation of chalcone derivatives, which serve as precursors to flavonoid natural products. The Journal of Organic Chemistry often features studies employing similar aromatic ketones in Michael addition reactions. Additionally, 3-fluoropropiophenone functions as a building block in the synthesis of agrochemical intermediates, though this application is outside the specified scope. The ketone functionality enables nucleophilic addition reactions, allowing for the formation of secondary metabolites with potential anticancer activities. Recent advances in organocatalysis have demonstrated the utility of this compound in asymmetric aldol reactions for the synthesis of chiral pharmaceutical intermediates.
Reviews
Write Your Own Review