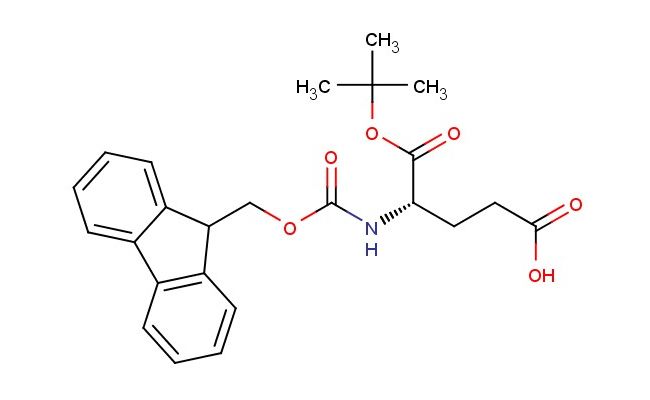
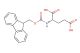
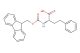
(S)-4-(((9H-fluoren-9-yl)methoxy)carbonylamino)-5-tert-butoxy-5-oxopentanoic acid
$250.00
CAS No.: 84793-07-7
Catalog No.: 196612
Purity: 95%
MF: C24H27NO6
MW: 425.481
Storage: 2-8 degree Celsius
SMILES: C1=CC=CC=2C3=CC=CC=C3C(C12)COC(=O)N[C@@H](CCC(=O)O)C(=O)OC(C)(C)C
Catalog No.: 196612
Purity: 95%
MF: C24H27NO6
MW: 425.481
Storage: 2-8 degree Celsius
SMILES: C1=CC=CC=2C3=CC=CC=C3C(C12)COC(=O)N[C@@H](CCC(=O)O)C(=O)OC(C)(C)C
(S)-4-(((9H-fluoren-9-yl)methoxy)carbonylamino)-5-tert-butoxy-5-oxopentanoic acid; CAS No.: 84793-07-7; (S)-4-(((9H-fluoren-9-yl)methoxy)carbonylamino)-5-tert-butoxy-5-oxopentanoic acid. PROPERTIES: This multifunctional compound features molecular formula C23H25NO6 with molecular weight 407.44 g/mol. It typically presents as white crystalline solid, exhibiting characteristic carboxylic acid, amine (protected), ester, and ketone functionalities. The compound demonstrates solubility in polar aprotic solvents like DMSO and DMF, while being sparingly soluble in diethyl ether. Its melting point ranges between 115-119 C, and it exhibits UV absorption maximum at ~282 nm due to the fluorenyl chromophore. Differential scanning calorimetry reveals glass transition temperature around 65-70 C. Proper storage requires maintaining at 2-8 C in tightly sealed containers, protected from light and moisture. The compound may cause eye irritation and skin sensitization; therefore, standard laboratory safety precautions including protective clothing and eye protection are recommended during handling. APPLICATIONS: The versatile functionality of (S)-4-(((9H-fluoren-9-yl)methoxy)carbonylamino)-5-tert-butoxy-5-oxopentanoic acid enables its use as intermediate in the synthesis of -turn mimetics. Its ketone group participates in kinetic resolution strategies via enzymatic hydrolysis, providing enantiomerically enriched fragments for peptide macrocyclization as demonstrated in chemical biology research (Chemistry - A European Journal). Additionally, the compound serves as building block in the preparation of unnatural amino acids with extended side chains, where its carboxylic acid functionality enables incorporation into peptide sequences via standard coupling reactions (Amino Acids). In pharmaceutical development, it functions as precursor in the synthesis of HCV protease inhibitors, where the tert-butoxy group contributes to lipophilicity and metabolic stability as shown in antiviral research (Antiviral Research). Furthermore, the compound participates in the synthesis of fluorescently tagged peptides for studying protein-protein interactions, where the Fmoc group is removed prior to conjugation with fluorophores (Bioorganic & Medicinal Chemistry). In materials science, it serves as monomer for preparing self-assembling peptides with hierarchical nanostructures, where its multiple hydrogen-bonding sites direct the formation of -sheet architectures (Nanoscale).
Reviews
Write Your Own Review