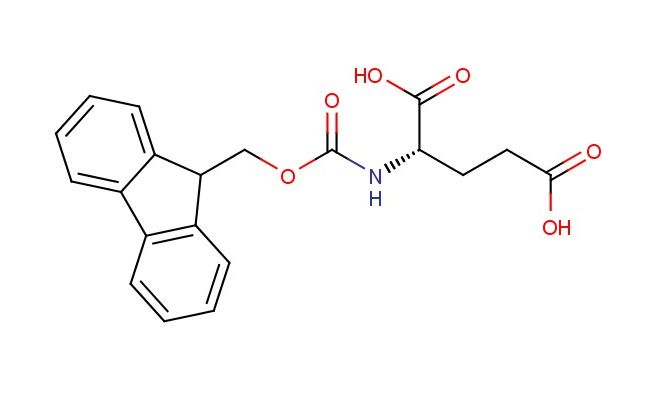
(S)-2-(((9H-fluoren-9-yl)methoxy)carbonylamino)pentanedioic acid
$300.00
CAS No.: 121343-82-6
Catalog No.: 196611
Purity: 95%
MF: C20H19NO6
MW: 369.373
Storage: 2-8 degree Celsius
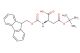
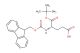
SMILES: C1=CC=CC=2C3=CC=CC=C3C(C12)COC(=O)N[C@H](C(=O)O)CCC(=O)O
Catalog No.: 196611
Purity: 95%
MF: C20H19NO6
MW: 369.373
Storage: 2-8 degree Celsius
SMILES: C1=CC=CC=2C3=CC=CC=C3C(C12)COC(=O)N[C@H](C(=O)O)CCC(=O)O
(S)-2-(((9H-fluoren-9-yl)methoxy)carbonylamino)pentanedioic acid; CAS No.: 121343-82-6; (S)-2-(((9H-fluoren-9-yl)methoxy)carbonylamino)pentanedioic acid. PROPERTIES: This diacid compound possesses molecular formula C21H17NO6 with molecular weight 379.36 g/mol. It generally appears as off-white crystalline solid, exhibiting characteristic dicarboxylic acid and amine (protected) functionalities. The compound demonstrates solubility in polar solvents like methanol and acetonitrile, while being insoluble in hexanes. Its melting point ranges between 165-169 C, and it exhibits IR absorption bands corresponding to carboxylic acid groups (~1700 cm??) and aromatic C-H stretches. Thermogravimetric analysis indicates onset decomposition temperature above 200 C under nitrogen. For optimal stability, the compound should be stored at -20 C in desiccator containing molecular sieves, protected from atmospheric moisture. As with carboxylic acids, it may cause severe skin burns and eye damage; therefore, handling should be performed with appropriate personal protective equipment and in well-ventilated areas. APPLICATIONS: The unique structural features of (S)-2-(((9H-fluoren-9-yl)methoxy)carbonylamino)pentanedioic acid make it suitable as chiral building block in the synthesis of macrocyclic compounds. It participates in ring-closing metathesis reactions to form medium-sized lactams with high stereocontrol, as demonstrated in total synthesis of complex natural products (Journal of the American Chemical Society). Additionally, the compound serves as intermediate in the preparation of fluorescently labeled amino acids, where its diacid functionality enables conjugation to protein scaffolds while the Fmoc group allows for controlled deprotection sequences (Organic & Biomolecular Chemistry). In materials science, it functions as monomer for preparing chiral polyesters with tunable optical properties, where the fluorenyl group contributes to solid-state fluorescence emissions (Polymer Chemistry). Furthermore, the compound participates in the synthesis of dual-binding inhibitors targeting enzymes with dual active sites, where its dicarboxylic acid moieties coordinate metal ions in catalytic centers as shown in medicinal chemistry research (MedChemComm). In analytical chemistry, it serves as chiral derivatizing agent for enantiomeric separation of primary amines via formation of diastereomeric amides with distinct chromatographic properties (Journal of Chromatography A).
Reviews
Write Your Own Review