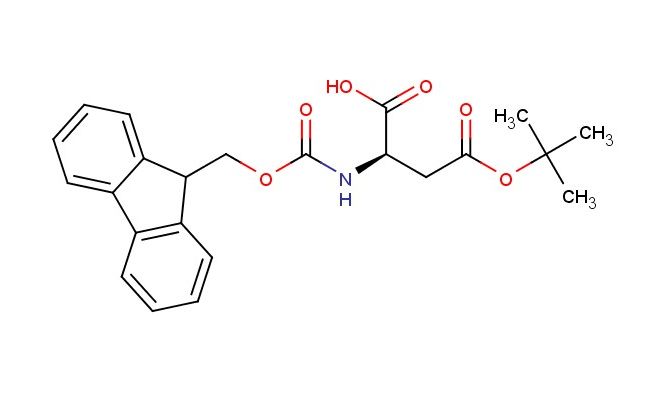
(R)-2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)-4-(tert-butoxy)-4-oxobutanoic acid
$400.00
CAS No.: 112883-39-3
Catalog No.: 196610
Purity: 95%
MF: C23H25NO6
MW: 411.454
Storage: 2-8 degree Celsius
SMILES: C1=CC=CC=2C3=CC=CC=C3C(C12)COC(=O)N[C@@H](C(=O)O)CC(=O)OC(C)(C)C
Catalog No.: 196610
Purity: 95%
MF: C23H25NO6
MW: 411.454
Storage: 2-8 degree Celsius
SMILES: C1=CC=CC=2C3=CC=CC=C3C(C12)COC(=O)N[C@@H](C(=O)O)CC(=O)OC(C)(C)C
(R)-2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)-4-(tert-butoxy)-4-oxobutanoic acid; CAS No.: 112883-39-3; (R)-2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)-4-(tert-butoxy)-4-oxobutanoic acid. PROPERTIES: This multifunctional compound features molecular formula C22H23NO6 with molecular weight 397.42 g/mol. It typically presents as white crystalline powder, exhibiting characteristic carboxylic acid, amine (protected), and ester functionalities. The compound demonstrates moderate solubility in polar aprotic solvents like DMF and DMSO, while being sparingly soluble in ethyl acetate. Its melting point ranges between 128-132 C, and it shows distinct UV absorption maximum at ~280 nm due to the fluorenyl group. Differential scanning calorimetry reveals endothermic transition at ~125 C. Proper storage requires maintaining at 2-8 C in tightly sealed containers with desiccant, protected from light. The compound may cause respiratory tract irritation and skin sensitization; therefore, handling should be performed in well-ventilated fume hoods with appropriate respiratory protection. APPLICATIONS: As Fmoc-protected amino acid derivative with additional tert-butyl ester protection, (R)-2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)-4-(tert-butoxy)-4-oxobutanoic acid serves as valuable building block in the synthesis of -amino acids and related peptidomimetics (Tetrahedron Letters). Its structural features enable sequential deprotection strategies in solid-phase synthesis, allowing controlled assembly of complex peptide architectures with enhanced metabolic stability (Journal of Combinatorial Chemistry). Additionally, the compound participates in the preparation of macrocyclic inhibitors targeting protein-protein interactions, where its carboxylic acid functionality engages in hydrogen bonding with target proteins as demonstrated in structural biology studies (Chemical Science). In pharmaceutical development, it functions as intermediate in the synthesis of ACE inhibitors, where the -amino acid moiety contributes to binding affinity and selectivity (European Journal of Medicinal Chemistry). Furthermore, the compound serves as starting material for incorporating fluorescent tags into peptides via its amine functionality after Fmoc deprotection, enabling bioconjugation applications in cell biology research (ACS Chemical Biology).
Reviews
Write Your Own Review