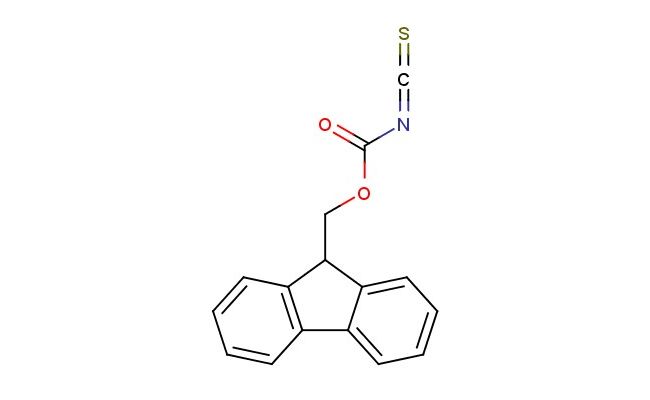
O-(9H-fluoren-9-yl)methyl carbonisothiocyanatidate
$300.00
CAS No.: 199915-38-3
Catalog No.: 196609
Purity: 95%
MF: C16H11NO2S
MW: 281.336
Storage: 2-8 degree Celsius
SMILES: C(OCC1C2=CC=CC=C2C=2C=CC=CC12)(=O)N=C=S
Catalog No.: 196609
Purity: 95%
MF: C16H11NO2S
MW: 281.336
Storage: 2-8 degree Celsius
SMILES: C(OCC1C2=CC=CC=C2C=2C=CC=CC12)(=O)N=C=S
O-(9H-fluoren-9-yl)methyl carbonisothiocyanatidate; CAS No.: 199915-38-3; O-(9H-fluoren-9-yl)methyl carbonisothiocyanatidate. PROPERTIES: This heterocumulene compound possesses molecular formula C14H10ON2S with molecular weight 266.30 g/mol. It generally appears as colorless to light yellow oily liquid, exhibiting characteristic reactivity of isothiocyanate and carbonate functionalities. The compound demonstrates solubility in common organic solvents like ethyl acetate and tetrahydrofuran, while being incompatible with strong nucleophiles. Its density is approximately 1.25 g/cm? at 20 C, and it exhibits distinct IR absorption bands corresponding to the isothiocyanate group (~2100 cm??) and aromatic C-H stretches. Thermogravimetric analysis indicates decomposition onset below 80 C, necessitating low-temperature handling. For stability, the compound should be stored at -20 C in amber glass bottles under nitrogen atmosphere, protected from moisture and heat. Due to its reactivity, it may cause severe skin burns and eye damage; therefore, rigorous containment and personal protection measures are essential during manipulation. APPLICATIONS: The dual functionalization of O-(9H-fluoren-9-yl)methyl carbonisothiocyanatidate enables its use as versatile crosslinking agent in polymer chemistry. It reacts with diols and diamines to form thiourea and carbonate crosslinks, imparting enhanced thermal stability to resulting polymeric networks as shown in macromolecular studies (Macromolecules). Additionally, the compound serves as key intermediate in the synthesis of fluorescent probes for bioimaging applications, where its isothiocyanate group reacts with primary amines in biomolecules while the fluorenyl moiety provides detectable fluorescence signal (Bioconjugate Chemistry). In pharmaceutical research, it functions as chemical modifier for introducing fluorescent labels into peptides and proteins, facilitating structural studies and interaction analyses (Analytical Chemistry). Furthermore, the compound participates in the preparation of thiourea-based organocatalysts, where its electronic properties influence enantioselectivity in asymmetric reactions (Advanced Synthesis & Catalysis).
Reviews
Write Your Own Review