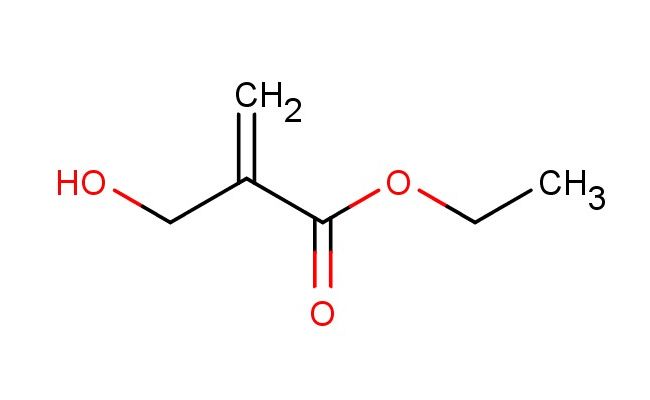
ethyl 2-(hydroxymethyl)acrylate
$300.00
CAS No.: 10029-04-6
Catalog No.: 196713
Purity: 95%
MF: C6H10O3
MW: 130.143
Storage: 2-8 degree Celsius
SMILES: OCC(C(=O)OCC)=C
Catalog No.: 196713
Purity: 95%
MF: C6H10O3
MW: 130.143
Storage: 2-8 degree Celsius
SMILES: OCC(C(=O)OCC)=C
ethyl 2-(hydroxymethyl)acrylate; CAS No.: 10029-04-6; ethyl 2-(hydroxymethyl)acrylate. PROPERTIES: This acrylate derivative features molecular formula C?H??O? with molecular weight 130.14 g/mol. It typically exists as a colorless liquid. Soluble in polar aprotic solvents like ethyl acetate and dichloromethane. Boiling point approximately 120-125 C. Exhibits IR absorption for ester (~1750 cm??) and hydroxyl groups (~3300 cm??). Thermogravimetric analysis indicates decomposition above 150 C under nitrogen. For optimal stability, ethyl 2-(hydroxymethyl)acrylate should be stored at 2-8 C in tightly sealed amber glass containers, protected from moisture and prolonged light exposure. As with acrylate compounds, it may cause severe skin burns and eye damage; therefore, rigorous containment and personal protection measures are essential during manipulation. APPLICATIONS: As a hydroxymethyl-substituted acrylate, ethyl 2-(hydroxymethyl)acrylate is predominantly utilized in the synthesis of polymeric materials. It serves as a key monomer in preparing hydrogels for biomedical applications, where its hydroxymethyl group provides valuable crosslinking sites for forming three-dimensional networks (Journal of Polymer Science). Additionally, the compound participates in the synthesis of surface-active agents, where its hydroxyl functionality enhances aqueous solubility and interfacial activity as demonstrated in colloid chemistry research (Langmuir). In pharmaceutical development, it functions as a building block for drug delivery systems, where its acrylate backbone facilitates controlled release of bioactive molecules (International Journal of Pharmaceutics). Furthermore, the compound serves as a starting material in the preparation of acrylate-based adhesives, where its hydroxyl group undergoes further modification to improve adhesion properties (Journal of Adhesion Science and Technology).
Reviews
Write Your Own Review