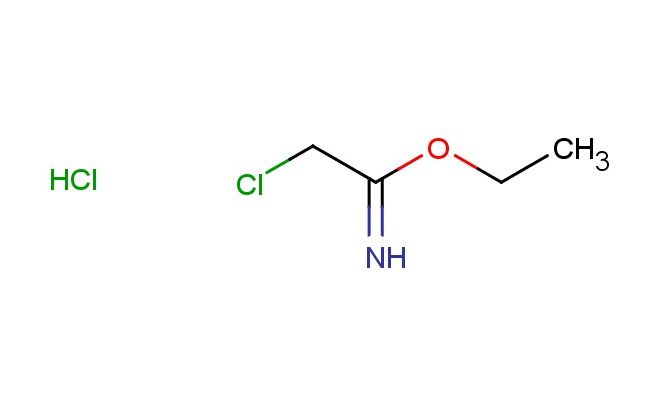
ethyl 2-chloroacetimidate hydrochloride
$200.00
CAS No.: 36743-66-5
Catalog No.: 196714
Purity: 95%
MF: C4H9Cl2NO
MW: 158.028
Storage: 2-8 degree Celsius
SMILES: Cl.ClCC(OCC)=N
Catalog No.: 196714
Purity: 95%
MF: C4H9Cl2NO
MW: 158.028
Storage: 2-8 degree Celsius
SMILES: Cl.ClCC(OCC)=N
ethyl 2-chloroacetimidate hydrochloride; CAS No.: 36743-66-5; ethyl 2-chloroacetimidate hydrochloride. PROPERTIES: This chlorinated acetimidate derivative features molecular formula C?H?Cl?NO with molecular weight 153.01 g/mol. It generally appears as a white crystalline powder. Soluble in polar protic solvents like methanol and water. Melting point approximately 150-155 C (decomposition). Exhibits IR absorption for imidate (~1650 cm??) and C-Cl groups (~600-500 cm??). Thermogravimetric analysis reveals weight loss onset above 120 C under nitrogen. For optimal stability, ethyl 2-chloroacetimidate hydrochloride should be stored at -20 C in desiccator containing molecular sieves, protected from atmospheric moisture. As with imidate compounds, it may cause severe skin burns and eye damage; therefore, rigorous containment and personal protection measures are essential during manipulation. APPLICATIONS: As a chlorinated acetimidate, ethyl 2-chloroacetimidate hydrochloride is particularly effective as a building block in nucleoside synthesis. It serves as a key intermediate in constructing modified nucleosides for antiviral agents, where the imidate group undergoes selective glycosylation reactions as demonstrated in medicinal chemistry research (Journal of Medicinal Chemistry). Additionally, the compound participates in the synthesis of fluorescent probes for bioimaging applications, where its chloride functionality enables conjugation to biomolecules via nucleophilic substitution reactions (Bioconjugate Chemistry). In materials science, it functions as a monomer for preparing polyimidate polymers with enhanced thermal stability, where the imidate group contributes to improved mechanical properties (Polymer Chemistry). Furthermore, the compound serves as a starting material in the development of imidate-based crosslinking agents for protein immobilization, where its reactive imidate group forms covalent bonds with amine-containing surfaces (ACS Applied Materials & Interfaces).
Reviews
Write Your Own Review