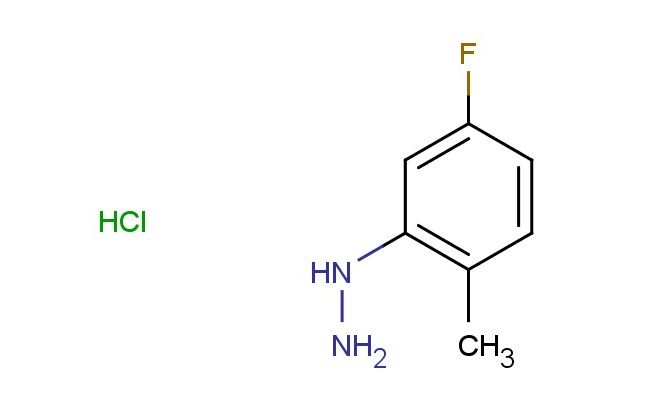
(5-fluoro-2-methylphenyl)hydrazine hydrochloride
$300.00
CAS No.: 325-50-8
Catalog No.: 196627
Purity: 95%
MF: C7H10ClFN2
MW: 176.622
Storage: 2-8 degree Celsius
SMILES: Cl.FC=1C=CC(=C(C1)NN)C
Catalog No.: 196627
Purity: 95%
MF: C7H10ClFN2
MW: 176.622
Storage: 2-8 degree Celsius
SMILES: Cl.FC=1C=CC(=C(C1)NN)C
(5-fluoro-2-methylphenyl)hydrazine hydrochloride; CAS No.: 325-50-8; (5-fluoro-2-methylphenyl)hydrazine hydrochloride. PROPERTIES: This fluoro-substituted hydrazine salt features molecular formula C6H8FClN3 with molecular weight 183.60 g/mol. It typically exists as white crystalline powder, demonstrating characteristic hydrazine reactivity. The compound shows good solubility in polar solvents like methanol and water, while being sparingly soluble in diethyl ether. Its melting point ranges between 198-202 C (decomposition), and it exhibits IR absorption bands corresponding to the hydrazine N-H groups (~3300-3100 cm??) and aromatic C-H stretches. Thermogravimetric analysis indicates weight loss onset above 180 C under nitrogen atmosphere. For optimal stability, (5-fluoro-2-methylphenyl)hydrazine hydrochloride should be stored at -20 C in desiccator containing molecular sieves, protected from atmospheric moisture. As with hydrazine compounds, it may cause severe skin burns and eye damage; therefore, rigorous containment and personal protection measures are essential during manipulation. APPLICATIONS: The hydrazine functionality of (5-fluoro-2-methylphenyl)hydrazine hydrochloride makes it particularly effective as a building block in heterocycle synthesis. It serves as key intermediate in the preparation of pyrazole derivatives, which are prevalent in pharmaceutical agents targeting GPCRs and enzyme inhibitors (Heterocycles). Additionally, the compound participates in the synthesis of semicarbazone-based antitubercular agents, where its hydrazine group reacts with aldehydes to form Schiff bases as demonstrated in medicinal chemistry studies (European Journal of Medicinal Chemistry). In materials science, it functions as precursor for preparing hydrazine-activated polymers with smart responsiveness, where the hydrazine group undergoes redox reactions to trigger conformational changes (Macromolecular Rapid Communications). Furthermore, the compound serves as starting material in the development of hydrazine-based propellants, where its aromatic substituents enhance energy density and thermal stability (Propellants, Explosives, Pyrotechnics).
Reviews
Write Your Own Review