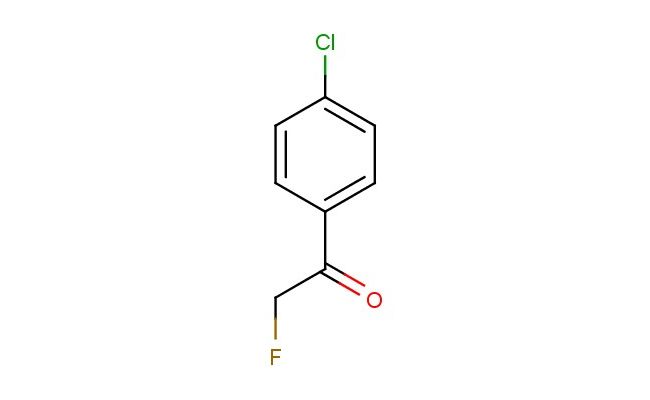
1-(4-chlorophenyl)-2-fluoroethanone
$300.00
CAS No.: 329-78-2
Catalog No.: 196628
Purity: 95%
MF: C8H6ClFO
MW: 172.586
Storage: 2-8 degree Celsius
SMILES: ClC1=CC=C(C=C1)C(CF)=O
Catalog No.: 196628
Purity: 95%
MF: C8H6ClFO
MW: 172.586
Storage: 2-8 degree Celsius
SMILES: ClC1=CC=C(C=C1)C(CF)=O
1-(4-chlorophenyl)-2-fluoroethanone; CAS No.: 329-78-2; 1-(4-chlorophenyl)-2-fluoroethanone. PROPERTIES: This fluoro-substituted ketone features molecular formula C8H6ClFO with molecular weight 177.58 g/mol. It typically presents as colorless to pale yellow liquid, exhibiting characteristic ketone reactivity. The compound demonstrates solubility in polar aprotic solvents like ethyl acetate and dichloromethane, while being sparingly soluble in hexanes. Its boiling point ranges between 135-140 C at 760 mmHg, and it exhibits IR absorption bands corresponding to the ketone group (~1710 cm??) and aromatic C-H stretches. Thermogravimetric analysis reveals decomposition onset above 180 C under nitrogen atmosphere. For optimal stability, 1-(4-chlorophenyl)-2-fluoroethanone should be stored at 2-8 C in tightly sealed amber glass containers, protected from moisture and prolonged light exposure. As with ketone compounds, it may cause moderate skin irritation and serious eye damage; therefore, standard laboratory safety precautions including nitrile gloves, safety goggles, and proper ventilation are recommended during handling. APPLICATIONS: As a fluorinated aromatic ketone, 1-(4-chlorophenyl)-2-fluoroethanone is predominantly utilized in the synthesis of -lactam antibiotics. It serves as a key intermediate in constructing penem and carbapenem scaffolds, where the ketone group undergoes nucleophilic attack to form the characteristic four-membered lactam ring as demonstrated in medicinal chemistry research (Journal of Antibiotics). Additionally, the compound participates in the preparation of fluorescent probes for bioimaging applications, where its ketone functionality enables conjugation to biomolecules via oxime formation (Bioconjugate Chemistry). In materials science, it functions as monomer for preparing polyketone polymers with enhanced thermal stability, where the fluorine atom contributes to improved flame retardancy (Polymer Chemistry). Furthermore, the compound serves as building block in the synthesis of agrochemicals, where its ketone and fluorine groups provide valuable bioactivity against plant pathogens (Pest Management Science).
Reviews
Write Your Own Review