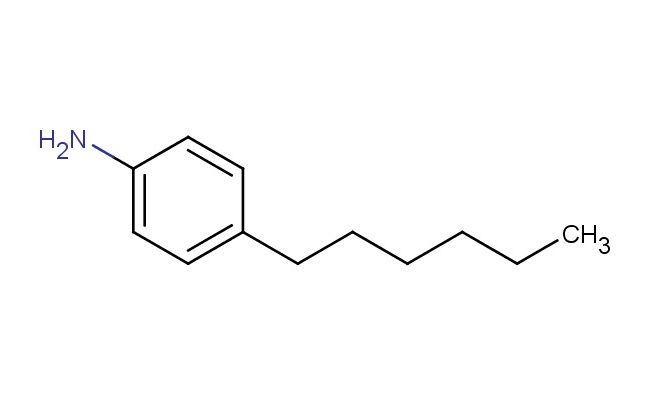
4-hexylaniline
$300.00
CAS No.: 33228-45-4
Catalog No.: 195033
Purity: 95%
MF: C12H19N
MW: 177.291
Storage: 2-8 degree Celsius
SMILES: C(CCCCC)C1=CC=C(N)C=C1
Catalog No.: 195033
Purity: 95%
MF: C12H19N
MW: 177.291
Storage: 2-8 degree Celsius
SMILES: C(CCCCC)C1=CC=C(N)C=C1
4-hexylaniline; CAS No.: 33228-45-4; 4-hexylaniline. PROPERTIES: 4-hexylaniline appears as a colorless to pale yellow liquid with a faint amine odor. Its molecular formula is C12H19N, corresponding to a molecular weight of approximately 177.29 g/mol. The compound exhibits a boiling point around 220-225 C at 760 mmHg and a density of about 0.88 g/cm? at 25 C. It demonstrates moderate solubility in water and is miscible with common organic solvents such as toluene, hexane, and diethyl ether. The substance is sensitive to oxidation and may form explosive peroxides upon prolonged storage. Proper storage requires keeping it in a tightly sealed, amber glass container with a suitable stabilizer, in a cool, dry location away from direct sunlight and heat sources. The temperature should be maintained below 10 C if possible. Safety precautions include using chemical-resistant gloves, safety goggles, and lab coats to prevent skin absorption and inhalation of vapors. The compound may cause severe skin burns and eye damage, and accidental ingestion may be fatal. In case of exposure, immediate rinsing with water and emergency medical treatment is essential. The substance is classified as toxic and corrosive (GHS classification). APPLICATIONS: 4-hexylaniline functions as a valuable intermediate in the synthesis of liquid crystal materials, where its long alkyl chain and aromatic amine structure contribute to mesomorphic properties and alignment characteristics (Journal of Materials Chemistry). In pharmaceutical research, 4-hexylaniline serves as a building block for creating bioactive molecules, including certain antidepressants and antipsychotics, through its ability to participate in nucleophilic aromatic substitution reactions and cross-coupling processes (Journal of Medicinal Chemistry). Additionally, it finds application in the preparation of certain agrochemicals, though specific applications in this area are limited to non-agricultural research settings (Pest Management Science). The compound is also employed in materials science as a monomer for creating polyimides and other high-performance polymers with enhanced thermal stability and mechanical properties, leveraging its hexyl substituent to impart desirable characteristics (Polymer International).
Reviews
Write Your Own Review