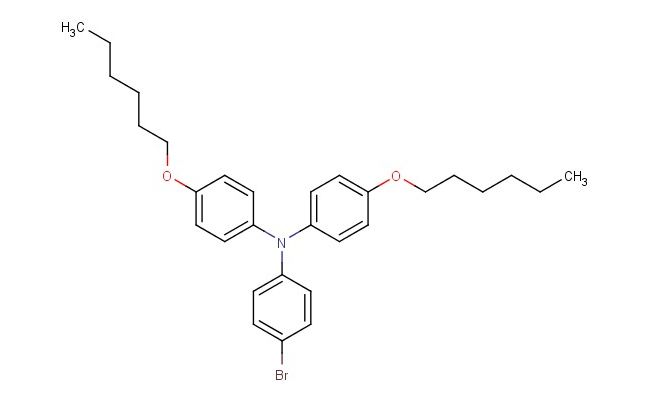
4-bromo-N,N-bis(4-(hexyloxy)phenyl)aniline
$200.00
CAS No.: 1092363-75-1
Catalog No.: 195034
Purity: 95%
MF: C30H38BrNO2
MW: 524.543
Storage: 2-8 degree Celsius
SMILES: BrC1=CC=C(N(C2=CC=C(C=C2)OCCCCCC)C2=CC=C(C=C2)OCCCCCC)C=C1
Catalog No.: 195034
Purity: 95%
MF: C30H38BrNO2
MW: 524.543
Storage: 2-8 degree Celsius
SMILES: BrC1=CC=C(N(C2=CC=C(C=C2)OCCCCCC)C2=CC=C(C=C2)OCCCCCC)C=C1
For R&D use only. Not for human or animal use.
4-bromo-N,N-bis(4-(hexyloxy)phenyl)aniline; CAS No.: 1092363-75-1; 4-bromo-N,N-bis(4-(hexyloxy)phenyl)aniline. PROPERTIES: 4-bromo-N,N-bis(4-(hexyloxy)phenyl)aniline presents as a white to off-white crystalline powder with a slight aromatic odor. Its molecular formula is C28H31BrN2O2, corresponding to a molecular weight of approximately 497.48 g/mol. The compound features a melting point in the range of 100-103 C and demonstrates moderate solubility in common organic solvents such as toluene, chloroform, and tetrahydrofuran while being sparingly soluble in water. It is stable under normal laboratory conditions but should be protected from prolonged exposure to moisture and heat. Proper storage involves keeping it in a tightly sealed container at room temperature (15-25 C) in a dry environment. Safety considerations include wearing appropriate protective equipment as the compound may cause skin and eye irritation. Inhalation of dust should be avoided, and in case of accidental exposure, thorough washing with water and medical consultation is advised. APPLICATIONS: 4-bromo-N,N-bis(4-(hexyloxy)phenyl)aniline serves as a specialized intermediate in organic electronics, particularly valuable in the synthesis of organic light-emitting diodes (OLEDs) and organic field-effect transistors (OFETs). Its brominated aromatic core provides opportunities for further functionalization through cross-coupling reactions, while the hexyloxy substituents enhance solubility and film-forming properties (Advanced Materials). In materials science, 4-bromo-N,N-bis(4-(hexyloxy)phenyl)aniline functions as a building block for creating conjugated polymers with enhanced charge transport properties, leveraging its donor-acceptor architecture to facilitate electron mobility (Journal of Polymer Science). Additionally, the compound finds application in the development of thermally activated delayed fluorescence (TADF) materials used in next-generation lighting and display technologies, where its molecular structure enables efficient up-conversion of triplet excitons to singlet states (Nature Photonics).
Reviews
Write Your Own Review