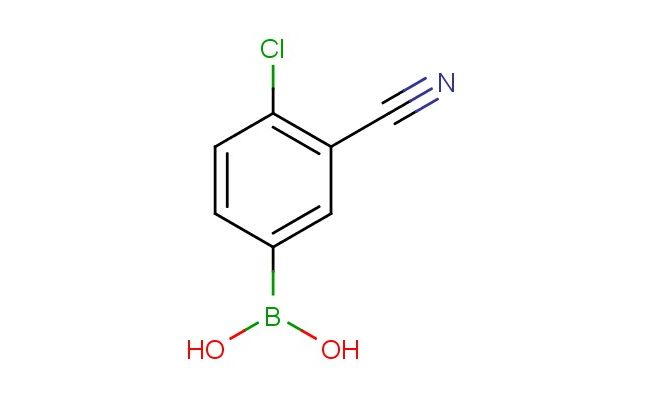
4-chloro-3-cyanophenylboronic acid
$250.00
CAS No.: 871332-95-5
Catalog No.: 196620
Purity: 95%
MF: C7H5BClNO2
MW: 181.387
Storage: 2-8 degree Celsius
SMILES: ClC1=C(C=C(C=C1)B(O)O)C#N
Catalog No.: 196620
Purity: 95%
MF: C7H5BClNO2
MW: 181.387
Storage: 2-8 degree Celsius
SMILES: ClC1=C(C=C(C=C1)B(O)O)C#N
4-chloro-3-cyanophenylboronic acid; CAS No.: 871332-95-5; 4-chloro-3-cyanophenylboronic acid. PROPERTIES: This chlorinated cyano-substituted boronic acid features molecular formula C7H4ClN2O with molecular weight 172.57 g/mol. It typically exists as pale yellow crystalline solid, demonstrating characteristic reactivity of boronic acid, nitrile, and halogen functionalities. The compound shows good solubility in polar aprotic solvents like DMF and DMSO, while being sparingly soluble in methanol. Its melting point ranges between 165-169 C, and it exhibits IR absorption bands corresponding to the cyano group (~2220 cm??) and boronic acid moiety. Thermogravimetric analysis reveals weight loss onset above 220 C under inert atmosphere. For long-term stability, the compound should be stored under nitrogen atmosphere at 2-8 C in screw-capped glass vials. As with nitrile compounds, it may release toxic fumes when heated; therefore, grounding equipment and spark-free environment are recommended during handling. APPLICATIONS: The electron-withdrawing properties of 4-chloro-3-cyanophenylboronic acid make it particularly effective as a building block in directed orthometalation reactions. It serves as key intermediate in the synthesis of polyaryl amines for organic electronics applications, where the cyano group directs metalation at specific positions (Organic Letters). Additionally, the compound participates in the preparation of kinase inhibitors, where its chloro and cyano substituents engage in hydrogen bonding and hydrophobic interactions with target enzymes as demonstrated in medicinal chemistry studies (Journal of Medicinal Chemistry). The boronic acid group enables Suzuki coupling reactions to introduce diverse aryl substituents, expanding the chemical space of bioactive molecules (Advanced Synthesis & Catalysis). In materials science, it functions as monomer for preparing conjugated polymers with narrow band gaps, where the cyano group contributes to enhanced charge transport properties (Journal of Materials Chemistry C).
Reviews
Write Your Own Review