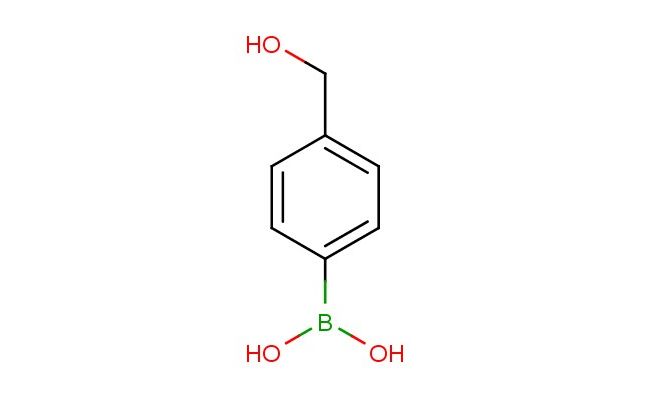
4-(hydroxymethyl)phenylboronic acid
$250.00
CAS No.: 59016-93-2
Catalog No.: 196619
Purity: 95%
MF: C7H9BO3
MW: 151.958
Storage: 2-8 degree Celsius
SMILES: OCC1=CC=C(C=C1)B(O)O
Catalog No.: 196619
Purity: 95%
MF: C7H9BO3
MW: 151.958
Storage: 2-8 degree Celsius
SMILES: OCC1=CC=C(C=C1)B(O)O
For R&D use only. Not for human or animal use.
4-(hydroxymethyl)phenylboronic acid; CAS No.: 59016-93-2; 4-(hydroxymethyl)phenylboronic acid. PROPERTIES: This hydroxymethyl-substituted boronic acid possesses molecular formula C7H7BO3 with molecular weight 152.03 g/mol. It generally appears as white crystalline powder, exhibiting characteristic boronic acid reactivity. The compound demonstrates solubility in polar protic solvents like water and methanol, while being insoluble in diethyl ether. Its melting point ranges between 128-132 C, and it shows distinct UV absorption maxima at 210-220 nm due to the aromatic system. Differential scanning calorimetry indicates thermal transition at ~120 C. Proper storage requires maintaining at -20 C in desiccator containing molecular sieves, away from atmospheric moisture. The compound may cause moderate skin irritation and serious eye damage; therefore, handling should be performed in well-ventilated areas with appropriate personal protective equipment. APPLICATIONS: As a sugar-binding boronic acid derivative, 4-(hydroxymethyl)phenylboronic acid is predominantly utilized in the synthesis of carbohydrate sensors. It forms reversible covalent complexes with diol-containing saccharides, enabling fluorescent or colorimetric detection as shown in chemical biology research (Chemical Communications). Additionally, the compound serves as intermediate in the preparation of glucose-responsive materials, where its boronic acid group coordinates with glucose in hydrogels to regulate drug release profiles (Biomaterials). In pharmaceutical development, it functions as a key building block for incorporating boronate moieties into targeting vectors for cancer therapy, where the hydroxymethyl group enhances aqueous solubility and tumor accumulation (Journal of Medicinal Chemistry). Furthermore, the compound participates in the synthesis of boronate affinity resins for protein purification, where its phenyl group improves chromatographic performance by reducing non-specific binding (Analytical Chemistry).
Reviews
Write Your Own Review